
Write only reactions for the preparation of benzophenone from benzonitrile.
Answer
592.2k+ views
Hint: The preparation of benzophenone from benzonitrile is a three-step process.
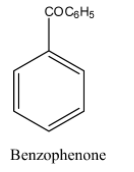
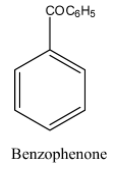
The structure of the benzophenone is as follows.
The structure of the benzonitrile is as follows.
Complete step by step solution:
Step-1:
In the step-1 hydrolysis of benzonitrile takes place. The chemical reaction of hydrolysis of benzonitrile is as follows.
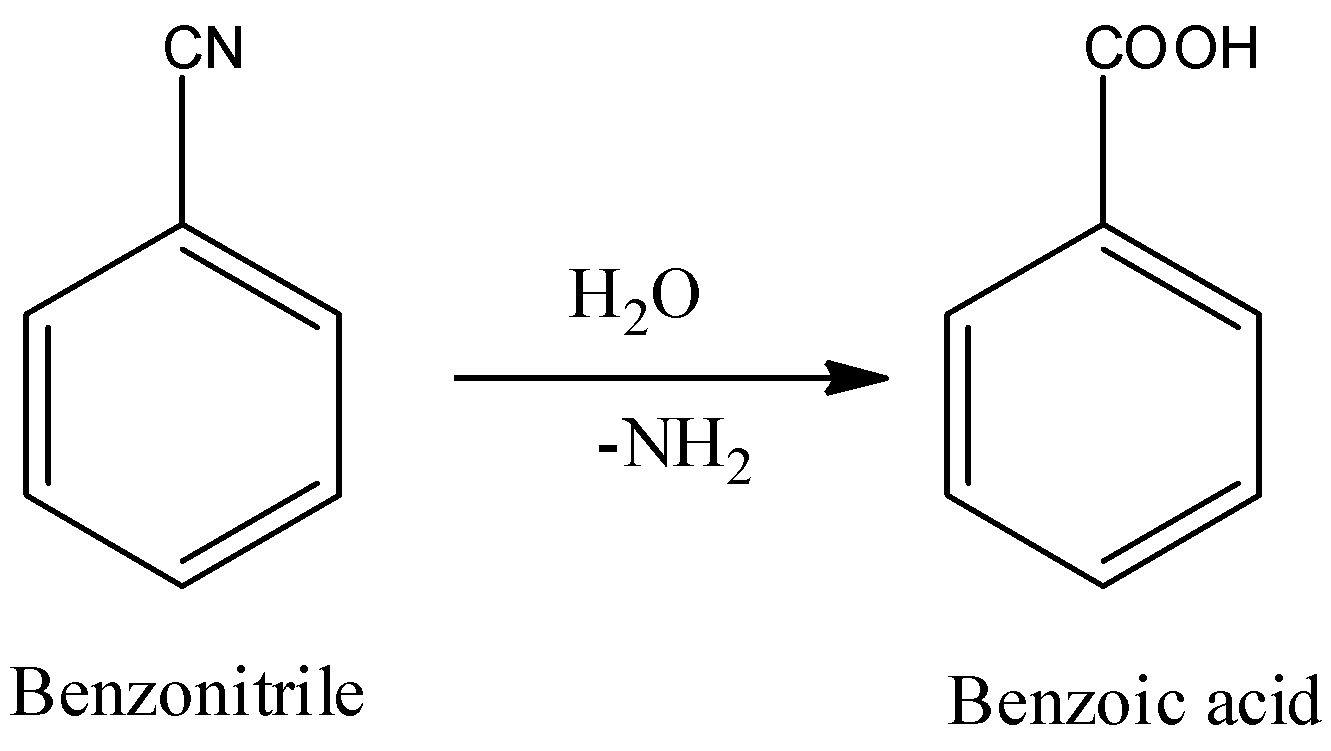
-In the above reaction benzonitrile undergoes hydrolysis and forms benzoic acid as the product.
-Generally, nitriles undergo hydrolysis and form carboxylic acids as the products.
Step-2:
In step-2 benzoic acid undergoes reaction with soda lime under heat and forms benzene as the product. The reaction of benzoic acid with soda lime is as follows.
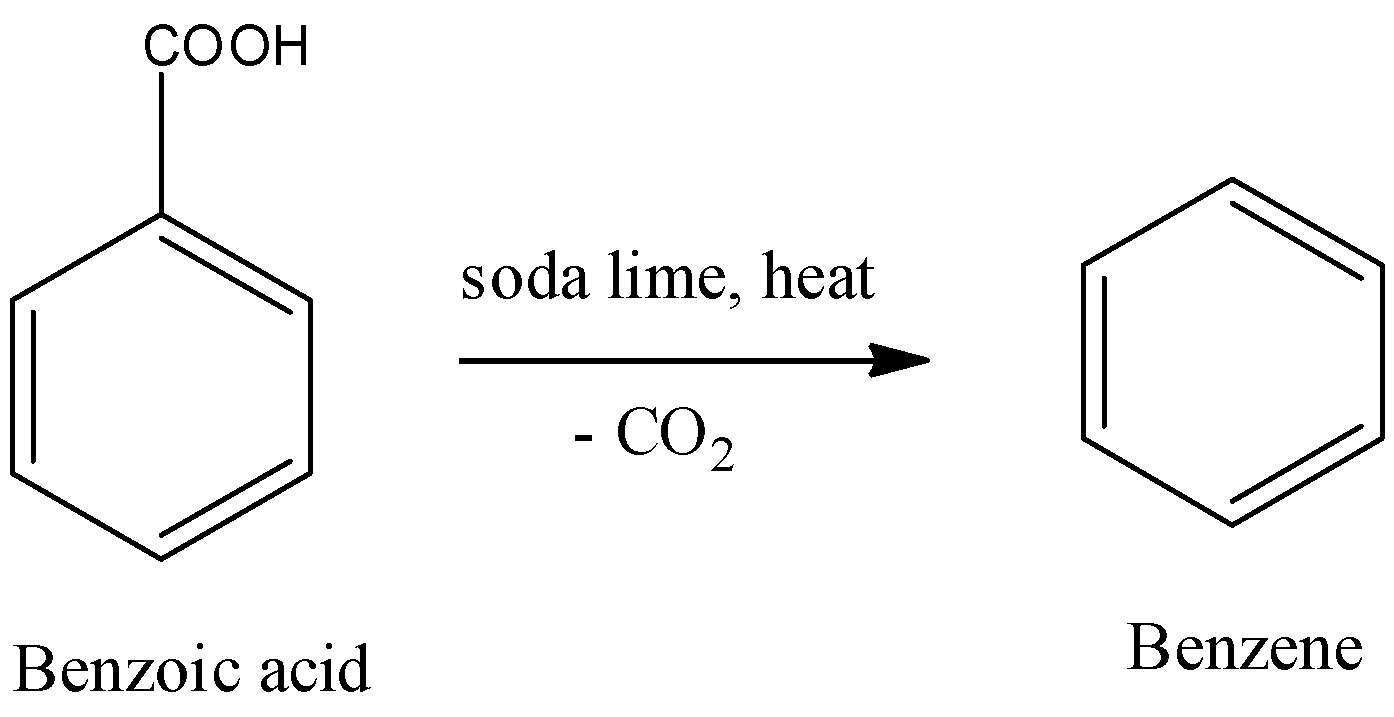
-The above reaction is called a decarboxylation reaction.
-A mixture of sodium hydroxide and calcium hydroxide is called soda lime.
Step-3:
-In step-3 benzene reacts with benzoyl chloride in presence of anhydrous aluminium chloride and forms benzophenone as the product.
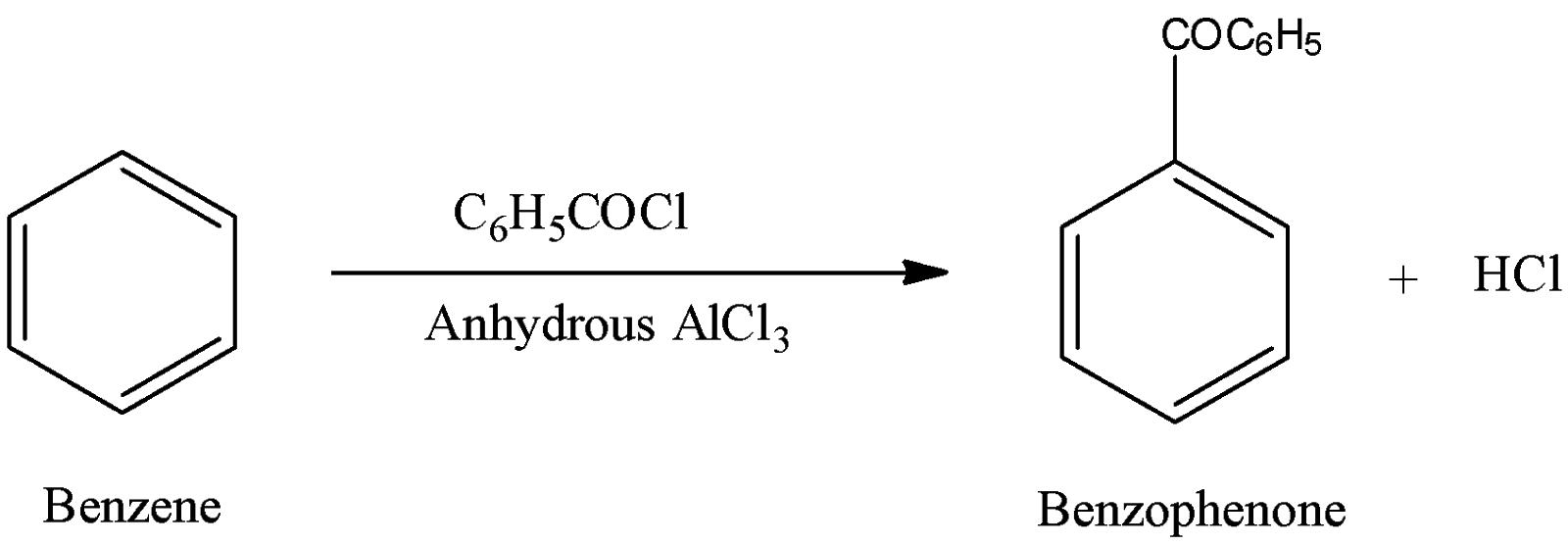
-The above reaction is an example of an electrophilic substitution reaction.
-The above reaction is called Friedel craft’s acylation reaction.
Additional information:
-Benzophenone is used as an additive for plastics.
-Perfumes, sunscreen lotions, nail polish, and shampoo contain benzophenone in their composition.
-The molecular formula of benzophenone is ${{C}_{13}}{{H}_{10}}O$.
-Benzophenone is a liquid and sweet-smelling organic compound in nature.
Note: Benzophenone in excess amounts is toxic. Generally, Cyanide compounds are called nitriles as per IUPAC nomenclature. We cannot prepare benzophenone from benzonitrile in a single-step reaction. Here we discussed the easiest way of preparation of benzophenone from benzonitrile.
The structure of the benzophenone is as follows.

The structure of the benzonitrile is as follows.

Complete step by step solution:
Step-1:
In the step-1 hydrolysis of benzonitrile takes place. The chemical reaction of hydrolysis of benzonitrile is as follows.

-In the above reaction benzonitrile undergoes hydrolysis and forms benzoic acid as the product.
-Generally, nitriles undergo hydrolysis and form carboxylic acids as the products.
Step-2:
In step-2 benzoic acid undergoes reaction with soda lime under heat and forms benzene as the product. The reaction of benzoic acid with soda lime is as follows.

-The above reaction is called a decarboxylation reaction.
-A mixture of sodium hydroxide and calcium hydroxide is called soda lime.
Step-3:
-In step-3 benzene reacts with benzoyl chloride in presence of anhydrous aluminium chloride and forms benzophenone as the product.

-The above reaction is an example of an electrophilic substitution reaction.
-The above reaction is called Friedel craft’s acylation reaction.
Additional information:
-Benzophenone is used as an additive for plastics.
-Perfumes, sunscreen lotions, nail polish, and shampoo contain benzophenone in their composition.
-The molecular formula of benzophenone is ${{C}_{13}}{{H}_{10}}O$.
-Benzophenone is a liquid and sweet-smelling organic compound in nature.
Note: Benzophenone in excess amounts is toxic. Generally, Cyanide compounds are called nitriles as per IUPAC nomenclature. We cannot prepare benzophenone from benzonitrile in a single-step reaction. Here we discussed the easiest way of preparation of benzophenone from benzonitrile.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




