
Write name of reaction.
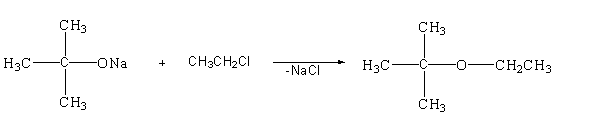
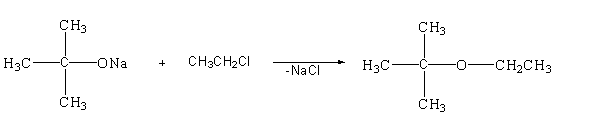
Answer
576.9k+ views
Hint:In the given reaction, the halide group present in chloro ethane which is the leaving group is replaced by the attacking species to form the desired product. The attacking takes place in the reaction from the back side of the carbon atom. The rate determining step depends on both the reacting species.
Complete step by step answer:
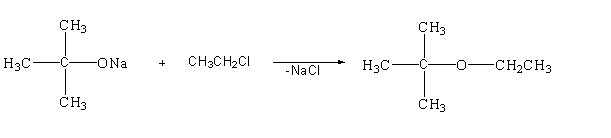
This reaction is a type of nucleophilic substitution reaction as it follows the ${S_N}2$ mechanism.
-In ${S_N}2$ reaction, the rate determining step depends on both the reacting species. It is a single step reaction. In this reaction the bond breaking and bond forming takes place synchronously. The methyl halide group and primary alkyl halide undergoes ${S_N}2$ fastly as compared to secondary halide and tertiary halide.
-In this reaction oxygen is the nucleophile and chloro group is the leaving group. The lone pair present in the oxygen atom will attack the alpha carbon from the back side of the reaction and will remove the chlorine atom and get attached to it. The chloro carrying the negative charge will again attack the sodium atom carrying the positive charge and will remove in the form of sodium chloride. The bond will now shift to form the desired product.
Therefore, the name of reaction is nucleophilic substitution reaction ${S_N}2$.
Note:
The ${S_N}2$ reaction is a type of stereospecific reaction, where different stereoisomers react with each other to form different stereoisomers of the product. In ${S_N}2$ reaction, Walden inversion takes place where a carbon atom which is asymmetric in nature experiences inversion of configuration.
Complete step by step answer:
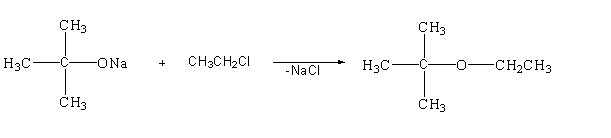
This reaction is a type of nucleophilic substitution reaction as it follows the ${S_N}2$ mechanism.
-In ${S_N}2$ reaction, the rate determining step depends on both the reacting species. It is a single step reaction. In this reaction the bond breaking and bond forming takes place synchronously. The methyl halide group and primary alkyl halide undergoes ${S_N}2$ fastly as compared to secondary halide and tertiary halide.
-In this reaction oxygen is the nucleophile and chloro group is the leaving group. The lone pair present in the oxygen atom will attack the alpha carbon from the back side of the reaction and will remove the chlorine atom and get attached to it. The chloro carrying the negative charge will again attack the sodium atom carrying the positive charge and will remove in the form of sodium chloride. The bond will now shift to form the desired product.
Therefore, the name of reaction is nucleophilic substitution reaction ${S_N}2$.
Note:
The ${S_N}2$ reaction is a type of stereospecific reaction, where different stereoisomers react with each other to form different stereoisomers of the product. In ${S_N}2$ reaction, Walden inversion takes place where a carbon atom which is asymmetric in nature experiences inversion of configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




