
Write Lewis structure of the $HN{O_3}$ and show formal charge on each atom.
Answer
565.5k+ views
Hint:We can say a Lewis Structure is an improved portrayal of the valence shell electrons in an atom. It is utilized to show how the electrons are organized around singular iotas in a particle. Electrons appear as "dots" or for holding electrons as a line between the two molecules. Lewis structures are known as electron dot structures, Lewis dot structures (or) Lewis electron dot structures.
Complete step by step answer:
Lewis Structure: We can say a Lewis structure shows a covalent bond as a pair of electrons divided among two molecules.
Procedure to write Lewis formula:
1.The symbols of the atoms which are attached together in the molecule next to one another are organized.
2.The total number of valence electrons in the molecule is obtained by adding the number of outermost electrons for all the atoms in the molecules. If the species is an ion, then the charge of an ion is considered by adding electrons, if it is a negative ion or subtracting electrons if it is a positive ion.
3.A two-electron covalent bond is indicated by placing a line between the atoms, which are assumed to be linked to each other.
4.The leftover valence electrons as lone pairs about each atom are organized so that the octet rule is satisfied for each other.
We can calculate the total number of valence electrons of nitric acid is,
Number of valence electrons in hydrogen= \[\left( 1 \right)\left( 1 \right) = 1\] electrons
Number of valence electrons in nitrogen=\[\left( 1 \right)\left( 5 \right) = 5\] electrons
Number of valence electrons in oxygen= $\left( 3 \right)\left( 6 \right) = 18$ electrons
The total number of valence electrons is twenty-four electrons.
We can draw the Lewis structure of nitric acid as,
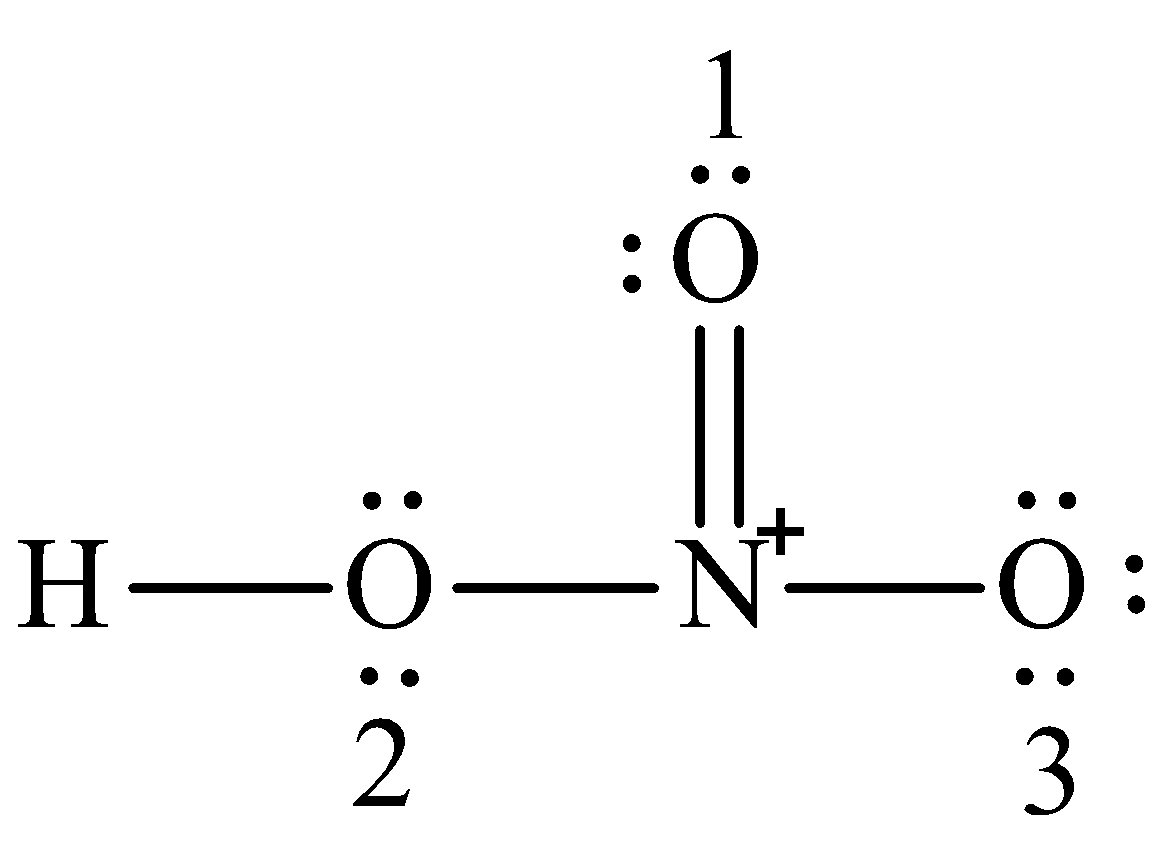
Formal charge (F.C): The charges that allot to every atom in a molecule (or) molecule by a bunch of self-assertive standards and do not necessarily indicate the actual charges on the molecules are called formal charges.
We could calculate the formal charge using the formula,
${\text{F}}{\text{.C}}{\text{.}} = {\text{Valence electrons}} - {\text{No}}{\text{. of non - bonding electrons}} - \dfrac{{{\text{No}}{\text{. of bonding electrons}}}}{2}$
We can say that the Lewis structure that contains zero formal charge or least separated formal charges is the preferred structure of the molecule.
Formal charge on oxygen (1) is $6 - 4 - \dfrac{1}{2}\left( 4 \right) = 0$
Formal charge on oxygen (2) is $6 - 4 - \dfrac{1}{2}\left( 4 \right) = 0$
Formal charge on oxygen (3) is $6 - 4 - \dfrac{1}{2}\left( 2 \right) = - 1$
Formal charge on nitrogen is $5 - 0 - \dfrac{1}{2}\left( 8 \right) = + 1$
Formal charge on hydrogen is $1 - 0 - \dfrac{1}{2}\left( 2 \right) = 0$
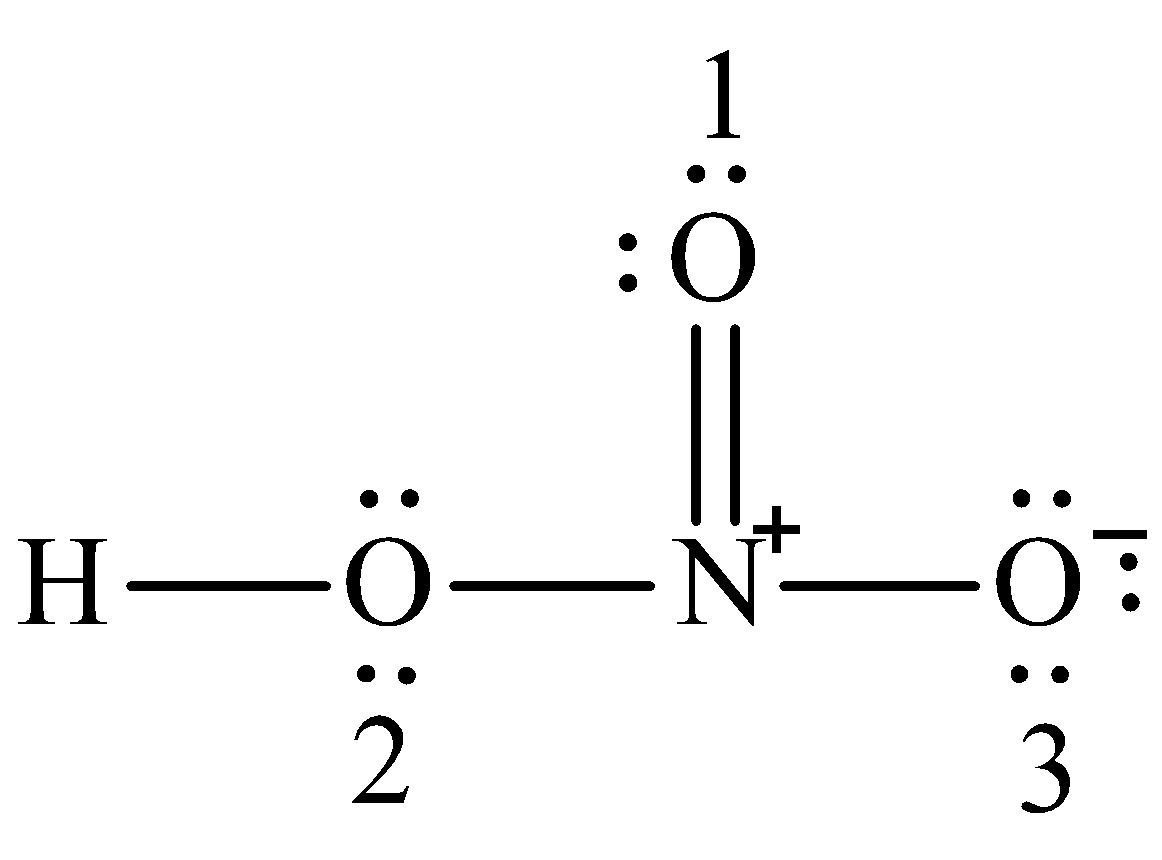
We can draw the Lewis structure of nitric acid is,
Note:
We could say that the formal charge of a molecule is figured as the contrast between the quantity of valence electrons that a neutral atom would have and the quantity of electrons that have a place with it in the Lewis structure. Electrons in covalent bonds are part similarly between the particles associated with the bond.
Complete step by step answer:
Lewis Structure: We can say a Lewis structure shows a covalent bond as a pair of electrons divided among two molecules.
Procedure to write Lewis formula:
1.The symbols of the atoms which are attached together in the molecule next to one another are organized.
2.The total number of valence electrons in the molecule is obtained by adding the number of outermost electrons for all the atoms in the molecules. If the species is an ion, then the charge of an ion is considered by adding electrons, if it is a negative ion or subtracting electrons if it is a positive ion.
3.A two-electron covalent bond is indicated by placing a line between the atoms, which are assumed to be linked to each other.
4.The leftover valence electrons as lone pairs about each atom are organized so that the octet rule is satisfied for each other.
We can calculate the total number of valence electrons of nitric acid is,
Number of valence electrons in hydrogen= \[\left( 1 \right)\left( 1 \right) = 1\] electrons
Number of valence electrons in nitrogen=\[\left( 1 \right)\left( 5 \right) = 5\] electrons
Number of valence electrons in oxygen= $\left( 3 \right)\left( 6 \right) = 18$ electrons
The total number of valence electrons is twenty-four electrons.
We can draw the Lewis structure of nitric acid as,

Formal charge (F.C): The charges that allot to every atom in a molecule (or) molecule by a bunch of self-assertive standards and do not necessarily indicate the actual charges on the molecules are called formal charges.
We could calculate the formal charge using the formula,
${\text{F}}{\text{.C}}{\text{.}} = {\text{Valence electrons}} - {\text{No}}{\text{. of non - bonding electrons}} - \dfrac{{{\text{No}}{\text{. of bonding electrons}}}}{2}$
We can say that the Lewis structure that contains zero formal charge or least separated formal charges is the preferred structure of the molecule.
Formal charge on oxygen (1) is $6 - 4 - \dfrac{1}{2}\left( 4 \right) = 0$
Formal charge on oxygen (2) is $6 - 4 - \dfrac{1}{2}\left( 4 \right) = 0$
Formal charge on oxygen (3) is $6 - 4 - \dfrac{1}{2}\left( 2 \right) = - 1$
Formal charge on nitrogen is $5 - 0 - \dfrac{1}{2}\left( 8 \right) = + 1$
Formal charge on hydrogen is $1 - 0 - \dfrac{1}{2}\left( 2 \right) = 0$
We can draw the Lewis structure of nitric acid is,

Note:
We could say that the formal charge of a molecule is figured as the contrast between the quantity of valence electrons that a neutral atom would have and the quantity of electrons that have a place with it in the Lewis structure. Electrons in covalent bonds are part similarly between the particles associated with the bond.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




