
How to write an equation for the preparation of the Grignard's Reagent starting from benzene?
Answer
525.9k+ views
Hint : We know that the haloalkanes, in the presence of the $s{{p}^{3}}$ or $s{{p}^{2}}$ hybridized carbon atoms in the aryl and vinyl halides, are introduced to magnesium metal and generate organomagnesium halides known as a Grignard reagent.
Complete Step By Step Answer:
At first, an alkyl or aryl halide needs to react with magnesium, to undergo a transformation of electrophilic alkyl halide into nucleophilic carbanion molecules. The Grignard reagent on reaction with orthoformates may lead to the formation of the aldehyde acetals formed from the displacement of the alkoxy group. In these types of reactions, the heterocyclic Grignard reagents from alkyl, aryl, vinyl, and other functional groups react with diethyl ether at room temperatures to yield orthoesters. The respective Grignard reaction with ester.
Therefore, all the different reactions that include the Grignard reagent need to be done in an arid environment. Often termed as water being bad for Grignard reactions in general, moisture may disrupt many crucial results if not taken proper care of. The other yielded compound, Mg (OH) Br (also known as the basic bromide) can be considered as an intermediate stage between magnesium bromide and magnesium hydroxide.
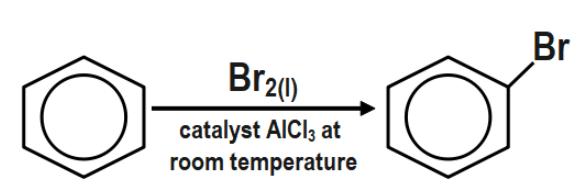
The first step is the bromination of benzene, and then you convert the bromobenzene to the Grignard reagent by reaction with magnesium in dry ether.
Note :
Remember that Since the Grignard reagents depend on the acidic nature of the functional groups, if the same is present in the halogen compounds, the Grignard reagent gets destroyed in the chemical process involving water, alcohol, or carboxylic acid groups.
Complete Step By Step Answer:
At first, an alkyl or aryl halide needs to react with magnesium, to undergo a transformation of electrophilic alkyl halide into nucleophilic carbanion molecules. The Grignard reagent on reaction with orthoformates may lead to the formation of the aldehyde acetals formed from the displacement of the alkoxy group. In these types of reactions, the heterocyclic Grignard reagents from alkyl, aryl, vinyl, and other functional groups react with diethyl ether at room temperatures to yield orthoesters. The respective Grignard reaction with ester.
Therefore, all the different reactions that include the Grignard reagent need to be done in an arid environment. Often termed as water being bad for Grignard reactions in general, moisture may disrupt many crucial results if not taken proper care of. The other yielded compound, Mg (OH) Br (also known as the basic bromide) can be considered as an intermediate stage between magnesium bromide and magnesium hydroxide.
The first step is the bromination of benzene, and then you convert the bromobenzene to the Grignard reagent by reaction with magnesium in dry ether.

Note :
Remember that Since the Grignard reagents depend on the acidic nature of the functional groups, if the same is present in the halogen compounds, the Grignard reagent gets destroyed in the chemical process involving water, alcohol, or carboxylic acid groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




