
Write a chemical test to distinguish between formaldehyde and acetaldehyde.
Answer
523.4k+ views
Hint: To distinguish one from another use reactions where a change of physical characteristics happens. For example, change of color of the solution, precipitation takes place, generation of effervescence, etc.
Complete step by step answer:
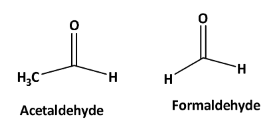
Structures of formaldehyde and acetaldehyde are
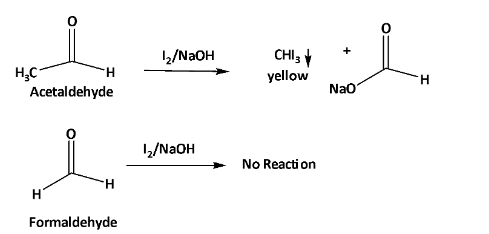
When formaldehyde and acetaldehyde are treated with iodine in NaOH. Acetaldehyde gives yellow color precipitation. But on the other hand, formaldehyde does not react with it.
From this reaction, a change of physical characteristic happens which can be visualized to understand the difference between formaldehyde and acetaldehyde.
The overall reaction is shown below.
The mechanism is shown below,
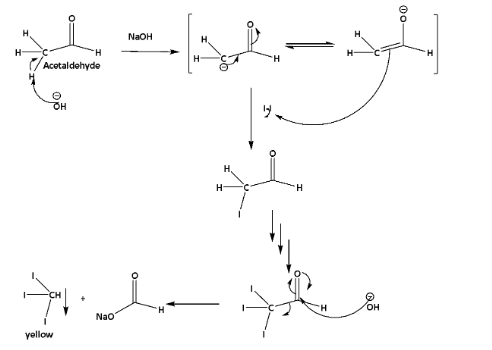
This reaction is commonly known as the iodoform reaction.
Iodoform reaction is used to prove the presence of \[R - CO - C{H_3}\] a group of carbonyl and \[R - CH\left( {OH} \right) - C{H_3}\] alcohol. In this reaction oxidation of the carbonyl group and alcohol group takes place. By oxidation, they form a carboxylic acid. And besides this, yellow precipitation of iodoform takes place in this reaction. This yellow precipitation is the indication that the reaction occurred.
Note:
When chlorine is used instead of iodine then it is called a chloroform reaction. In this reaction precipitation of white chloroform is formed. And when bromine is used instead of iodine or chlorine then it is called a bromoform reaction. In this reaction precipitation of pale yellow bromoform is formed. These types of reactions are collectively known as haloform reactions.
Complete step by step answer:
Structures of formaldehyde and acetaldehyde are

When formaldehyde and acetaldehyde are treated with iodine in NaOH. Acetaldehyde gives yellow color precipitation. But on the other hand, formaldehyde does not react with it.
From this reaction, a change of physical characteristic happens which can be visualized to understand the difference between formaldehyde and acetaldehyde.
The overall reaction is shown below.

The mechanism is shown below,

This reaction is commonly known as the iodoform reaction.
Iodoform reaction is used to prove the presence of \[R - CO - C{H_3}\] a group of carbonyl and \[R - CH\left( {OH} \right) - C{H_3}\] alcohol. In this reaction oxidation of the carbonyl group and alcohol group takes place. By oxidation, they form a carboxylic acid. And besides this, yellow precipitation of iodoform takes place in this reaction. This yellow precipitation is the indication that the reaction occurred.
Note:
When chlorine is used instead of iodine then it is called a chloroform reaction. In this reaction precipitation of white chloroform is formed. And when bromine is used instead of iodine or chlorine then it is called a bromoform reaction. In this reaction precipitation of pale yellow bromoform is formed. These types of reactions are collectively known as haloform reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




