
Write a brief note on hyperconjugation and give all hyper conjugated structures of propene.
Answer
599.7k+ views
Hint- Proceed the solution of this question using the concept of hyper-conjugation, which is a special case of resonance that involves delocalisation of σ electrons of C−H bond of any alkyl group. Hence, in the same way show the delocalisation of σ electrons of C−H bond in propene structure.
Complete answer:
Hyper-conjugation is the interaction of the electrons in a σ bond with an adjacent empty or partially filled non-bonding p-orbital, antibonding σ or π orbital or filled π orbital to give an extended molecular orbital that increases the stability of the system.
In case of propene, hyper conjugation arise due to partial overlap of ${\text{s}}{{\text{p}}^3}$ sigma bond orbital and the empty p-orbital of an adjacent c−atom.
Here one of the C-atom C−H bonds of $ - {\text{C}}{{\text{H}}_3}$ group can lie in the plane of pi-bond orbital, hence partial overlapping.
Hyper conjugation structures of propene are given below-
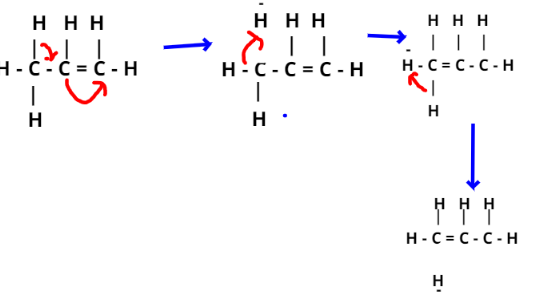
Note- In this particular question, we should know that Hyperconjugation is also known as no bond resonance. You may note that resonance involves delocalisation of π electrons but it’s the σ electrons that are delocalised in hyper conjugation. The normal carbon-carbon single bond length is \[1.54{\text{ }}{{\text{A}}^0}\]. However, in some compounds such as propene, it is a little shorter because of presence of some double bond character in C−C single bond due to hyper conjugation
Complete answer:
Hyper-conjugation is the interaction of the electrons in a σ bond with an adjacent empty or partially filled non-bonding p-orbital, antibonding σ or π orbital or filled π orbital to give an extended molecular orbital that increases the stability of the system.
In case of propene, hyper conjugation arise due to partial overlap of ${\text{s}}{{\text{p}}^3}$ sigma bond orbital and the empty p-orbital of an adjacent c−atom.
Here one of the C-atom C−H bonds of $ - {\text{C}}{{\text{H}}_3}$ group can lie in the plane of pi-bond orbital, hence partial overlapping.
Hyper conjugation structures of propene are given below-

Note- In this particular question, we should know that Hyperconjugation is also known as no bond resonance. You may note that resonance involves delocalisation of π electrons but it’s the σ electrons that are delocalised in hyper conjugation. The normal carbon-carbon single bond length is \[1.54{\text{ }}{{\text{A}}^0}\]. However, in some compounds such as propene, it is a little shorter because of presence of some double bond character in C−C single bond due to hyper conjugation
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




