
With the reference of the scheme given, which of the given statement (s) about T, U, V and W is correct?
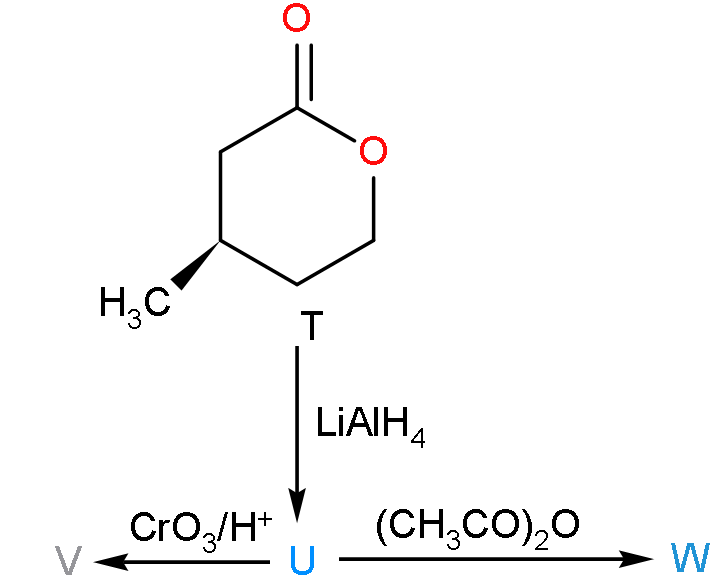
A. T is soluble in hot aqueous NaOH
B. U is optically active
C. Molecular formula of W is ${{\text{C}}_{10}}{{\text{H}}_{18}}{{\text{O}}_{4}}$
D. V gives effervescence on treatment with $\text{NaHC}{{\text{O}}_{3}}$

Answer
579.9k+ views
Hint: For this problem, firstly we have to write the product which will be formed when the compound T will react with Lithium aluminium hydroxide and then the further reaction to write the products V and W so that we can determine all the options.
Complete step by step answer:
- In the given question, we have to explain that among the given options which option is correct or not.
- Now, firstly the compound T will reduce when it will react with lithium aluminium hydroxide because it is a reducing agent so the product formed will be:
- Now, here the compound T when placed in the alkaline medium of sodium hydroxide then it will form a salt complex due to which it will be soluble.
- So, we can say that the compound T will be soluble in the hot aqueous solution of the sodium hydroxide.
- Now, when the compound U will react with acetic anhydride with highly acidic because it consists of two molecules of acetic acid.
- Then it will yield a compound as shown below:
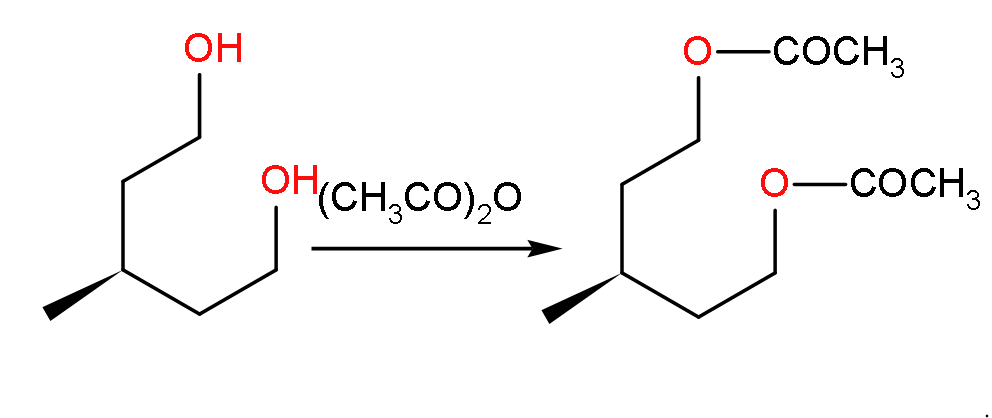
- So, here the product formed will have the molecular formula of ${{\text{C}}_{10}}{{\text{H}}_{18}}{{\text{O}}_{4}}$ because we can see in the structure that there are 10 carbon atoms, 18 hydrogen atoms and 4 oxygen atoms.
- So, we can say the statement C is correct.
- Now, firstly we have to find out the product V which is formed when the molecule U will undergo oxidation due to the presence of $\text{Cr}{{\text{O}}_{3}}/{{\text{H}}^{+}}$.
- So the product formed will be:
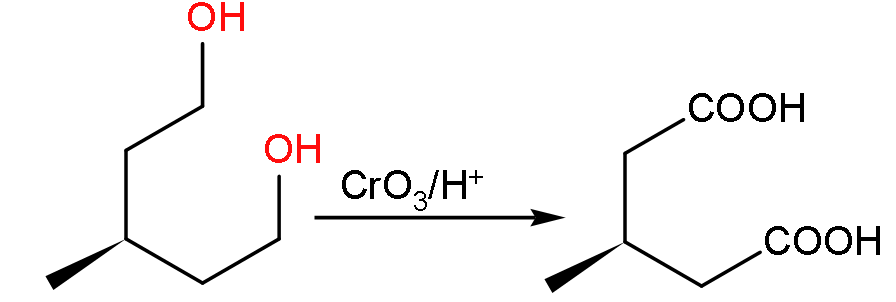
- Now, the molecular formula of the compound V will be ${{\text{C}}_{6}}{{\text{H}}_{10}}{{\text{O}}_{4}}$ so when it reacts with sodium carbonate it gives carbon dioxide gas.
- Due to the production of carbon dioxide gas it will show the effervescence as shown below:
${{\text{C}}_{6}}{{\text{H}}_{10}}{{\text{O}}_{4}}\text{ + NaHC}{{\text{O}}_{3}}\text{ }\to \text{ C}{{\text{O}}_{2}}\,\uparrow $
So, the correct answer is “Option A, C and D”.
Note: In this problem, the compound U is not the optically active compound because there is no chiral carbon present. As we know that the chiral carbon is the carbon which is attached to the four different groups.
Complete step by step answer:
- In the given question, we have to explain that among the given options which option is correct or not.
- Now, firstly the compound T will reduce when it will react with lithium aluminium hydroxide because it is a reducing agent so the product formed will be:
- Now, here the compound T when placed in the alkaline medium of sodium hydroxide then it will form a salt complex due to which it will be soluble.
- So, we can say that the compound T will be soluble in the hot aqueous solution of the sodium hydroxide.
- Now, when the compound U will react with acetic anhydride with highly acidic because it consists of two molecules of acetic acid.
- Then it will yield a compound as shown below:

- So, here the product formed will have the molecular formula of ${{\text{C}}_{10}}{{\text{H}}_{18}}{{\text{O}}_{4}}$ because we can see in the structure that there are 10 carbon atoms, 18 hydrogen atoms and 4 oxygen atoms.
- So, we can say the statement C is correct.
- Now, firstly we have to find out the product V which is formed when the molecule U will undergo oxidation due to the presence of $\text{Cr}{{\text{O}}_{3}}/{{\text{H}}^{+}}$.
- So the product formed will be:

- Now, the molecular formula of the compound V will be ${{\text{C}}_{6}}{{\text{H}}_{10}}{{\text{O}}_{4}}$ so when it reacts with sodium carbonate it gives carbon dioxide gas.
- Due to the production of carbon dioxide gas it will show the effervescence as shown below:
${{\text{C}}_{6}}{{\text{H}}_{10}}{{\text{O}}_{4}}\text{ + NaHC}{{\text{O}}_{3}}\text{ }\to \text{ C}{{\text{O}}_{2}}\,\uparrow $
So, the correct answer is “Option A, C and D”.
Note: In this problem, the compound U is not the optically active compound because there is no chiral carbon present. As we know that the chiral carbon is the carbon which is attached to the four different groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




