
With conc. ${\rm{HBr}}$ ethylphenyl ether yields;
A. Phenol and ethyl bromide
B. Bromobenzene and ethanol
C. Phenol and ethane
D. Bromobenzene and ethane
Answer
568.8k+ views
Hint: Alkyl ethers cleaved by strong acids such as ${\rm{HBr}}\;{\rm{and}}\;{\rm{HI}}$ in nucleophilic substitution reaction. The protonation of ethereal oxygen produces a good leaving group along with the neutral alcohol molecule. Depending on the structure of the alkyl group, the reaction can be ${\rm{S}}{{\rm{N}}_{\rm{2}}}$.
Complete step by step answer:
The ether molecules have net dipole moment due to the polarity ${\rm{C}} - {\rm{O}}$ bon d.The boiling points of ethers are comparable to alkanes but much lower than alcohols of comparable molecular masses. Ether molecules are miscible in water due to their polar nature (‘‘like dissolves like’’ rule i.e. polar molecule dissolves in polar solvent and nonpolar molecule will be dissolved in nonpolar solvent).
Diethyl ether used as a solvent in organic chemistry. Vapours of some ethers used as miticides, insecticides and fumigants for soil.
Ethyl phenyl ether reacts with conc. ${\rm{HBr}}$ to give alkyl halide and alcohol. The mechanism involves two steps as:
Step 1: Protonation of the alcoholic oxygen takes place to make a better leaving group. This step is very reversible and fast.
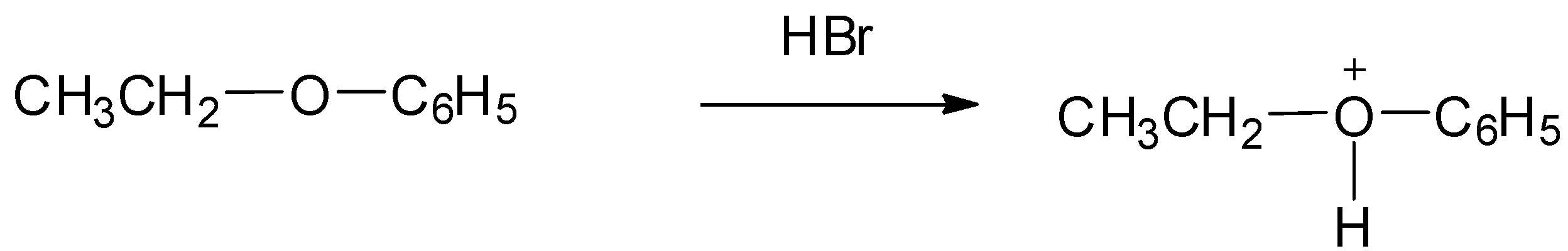
Step 2: The bromide ion acts as nucleophile and attacks to displace the good leaving by cleaving the ${\rm{C}} - {\rm{O}}$ bond producing neutral alcohol and alkyl bromide.
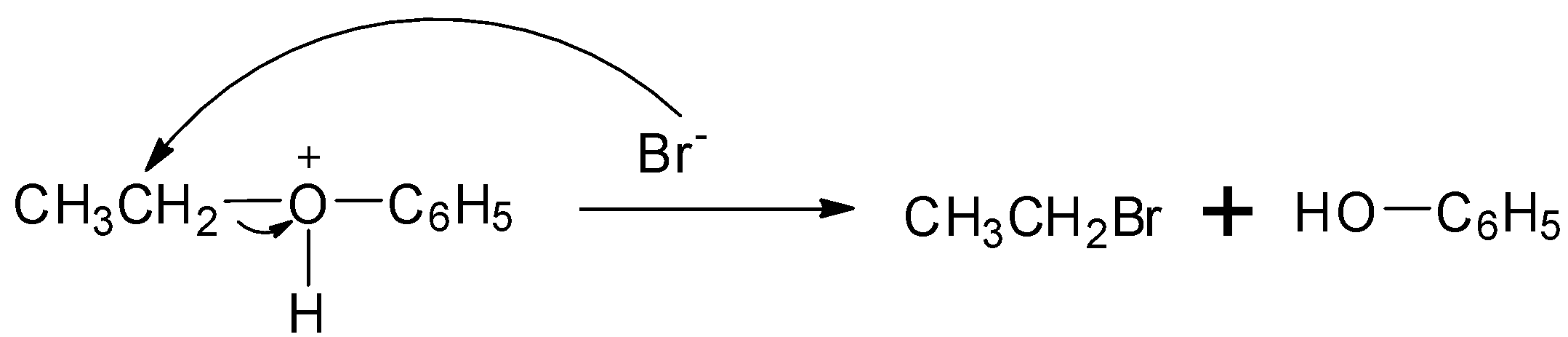
So, the correct answer is Option A.
Note: The leaving group in the above mechanism must be stable. In the above case, the second step is the slowest step and rate determining step. ${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}^{\rm{ + }}$ carbocation is more stable than ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}^{\rm{ + }}$ because the positive charge in ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}^{\rm{ + }}$ is localized over the ring.
Complete step by step answer:
The ether molecules have net dipole moment due to the polarity ${\rm{C}} - {\rm{O}}$ bon d.The boiling points of ethers are comparable to alkanes but much lower than alcohols of comparable molecular masses. Ether molecules are miscible in water due to their polar nature (‘‘like dissolves like’’ rule i.e. polar molecule dissolves in polar solvent and nonpolar molecule will be dissolved in nonpolar solvent).
Diethyl ether used as a solvent in organic chemistry. Vapours of some ethers used as miticides, insecticides and fumigants for soil.
Ethyl phenyl ether reacts with conc. ${\rm{HBr}}$ to give alkyl halide and alcohol. The mechanism involves two steps as:
Step 1: Protonation of the alcoholic oxygen takes place to make a better leaving group. This step is very reversible and fast.

Step 2: The bromide ion acts as nucleophile and attacks to displace the good leaving by cleaving the ${\rm{C}} - {\rm{O}}$ bond producing neutral alcohol and alkyl bromide.

So, the correct answer is Option A.
Note: The leaving group in the above mechanism must be stable. In the above case, the second step is the slowest step and rate determining step. ${\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}^{\rm{ + }}$ carbocation is more stable than ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}^{\rm{ + }}$ because the positive charge in ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{5}}}^{\rm{ + }}$ is localized over the ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




