
What is Williamson’s ether synthesis? Give an equation.
Answer
546.3k+ views
Hint: In this reaction, an ether is formed from an organohalide. The mechanism used in this reaction is \[{S_N}2\] mechanism.
Complete answer:
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol. This method is suitable for the preparation of a wide variety of unsymmetrical ethers. The reaction was discovered by Alexander William in 1850. It involves the attack of alkoxide ion on a primary halide via \[{S_N}2\] mechanism. This reaction is very important in the history of organic chemistry because it helped to prove the structure of ethers. However, If the halides are sterically demanding and there are accessible protons in the β-position, the alkoxide will act as a base, and side products derived from elimination are isolated instead.
The mechanism for Williamson synthesis is:
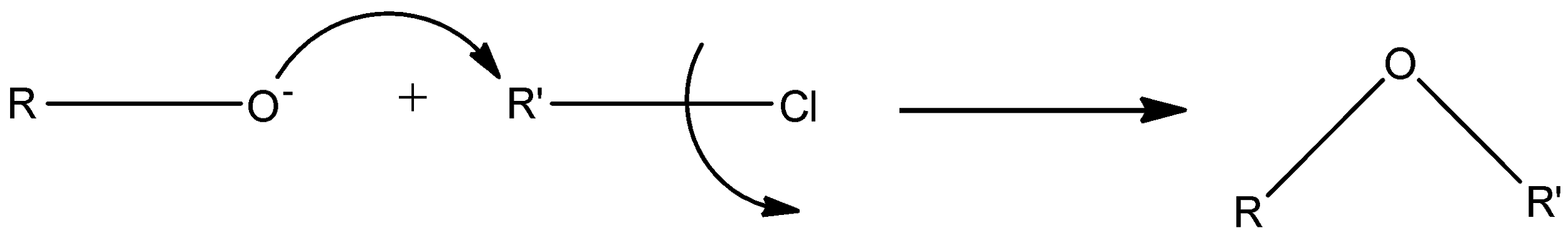
An example of Williamson ether synthesis is:
\[{C_2}{H_5}{O^ - }N{a^ + } + {C_2}{H_5}Cl \to {C_2}{H_5}O{C_2}{H_5} + N{a^ + }C{l^\_}\]
The Williamson ether synthesis is an \[{S_N}2\] bimolecular nucleophilic substitution reaction. In an \[{S_N}2\] reaction, the nucleophile attacks the anti-bonding orbital of the electrophile. It occurs in a concerted mechanism (all at once). The rate of reaction of an \[{S_N}2\] reaction depends mainly on the leaving tendency of the leaving group. The leaving group must be sufficiently electronegative, like a halide. In Williamson ether synthesis, alkoxide ion (\[R{O^ - }\]) acts as the nucleophile which attacks the electrophilic carbon with the leaving group.
Note:
Remember that the reaction follows \[{S_N}2\] mechanism. \[{S_N}2\] mechanism is a concerted mechanism, therefore finally an inverted product is formed.
Complete answer:
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol. This method is suitable for the preparation of a wide variety of unsymmetrical ethers. The reaction was discovered by Alexander William in 1850. It involves the attack of alkoxide ion on a primary halide via \[{S_N}2\] mechanism. This reaction is very important in the history of organic chemistry because it helped to prove the structure of ethers. However, If the halides are sterically demanding and there are accessible protons in the β-position, the alkoxide will act as a base, and side products derived from elimination are isolated instead.
The mechanism for Williamson synthesis is:

An example of Williamson ether synthesis is:
\[{C_2}{H_5}{O^ - }N{a^ + } + {C_2}{H_5}Cl \to {C_2}{H_5}O{C_2}{H_5} + N{a^ + }C{l^\_}\]
The Williamson ether synthesis is an \[{S_N}2\] bimolecular nucleophilic substitution reaction. In an \[{S_N}2\] reaction, the nucleophile attacks the anti-bonding orbital of the electrophile. It occurs in a concerted mechanism (all at once). The rate of reaction of an \[{S_N}2\] reaction depends mainly on the leaving tendency of the leaving group. The leaving group must be sufficiently electronegative, like a halide. In Williamson ether synthesis, alkoxide ion (\[R{O^ - }\]) acts as the nucleophile which attacks the electrophilic carbon with the leaving group.
Note:
Remember that the reaction follows \[{S_N}2\] mechanism. \[{S_N}2\] mechanism is a concerted mechanism, therefore finally an inverted product is formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




