
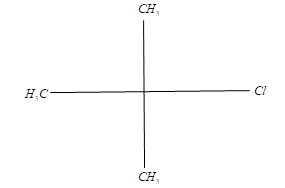
Will it undergo Wurtz reaction?

Answer
601.2k+ views
Hint: In order to deal with this question first we have to define the wurtz reaction and we will see its limitations further according to the condition we will comment whether the reaction happens or not.
Complete step by step solution:
Wurtz reaction is an organic chemical coupling reaction in which sodium metal is reacted in an atmosphere using two alkyl halides given by dry ether solution to create a higher alkane with sodium and halogen compounds.
Limitations of the Wurtz Reaction
A few limitations of this reaction are listed below.
Commonly, only symmetric alkanes can be synthesized via this method since a mixture of alkane products are formed when dissimilar alkanes are reacted (these mixtures are difficult to separate).
An alkene intermediate is formed by a side reaction. Should the alkyl halides be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed.
Methane cannot be synthesized by the Wurtz reaction, since at least two carbon atoms must occur as a part of an organic coupling reaction.
When tertiary alkyl halides are used, the Wurtz coupling process normally fails.
So, in Wurtz reaction, sodium reacts with alkyl halides in the presence of ether to form corresponding alkane. Tertiary halides having bulky groups undergo side reactions due to steric hindrance which results in an elimination reaction.
Hence, the given compound will have a very high tendency of undergoing elimination and hence will not undergo Wurtz reaction.
Note: Wurtz reaction is not preferred for preparation of alkane containing odd numbers of carbon atoms because in wurtz reaction the reactants are two different alkyl halides and due to which a mixture of alkanes is formed in the product. Also methane cannot be prepared using a wurtz reaction.
Complete step by step solution:
Wurtz reaction is an organic chemical coupling reaction in which sodium metal is reacted in an atmosphere using two alkyl halides given by dry ether solution to create a higher alkane with sodium and halogen compounds.
Limitations of the Wurtz Reaction
A few limitations of this reaction are listed below.
Commonly, only symmetric alkanes can be synthesized via this method since a mixture of alkane products are formed when dissimilar alkanes are reacted (these mixtures are difficult to separate).
An alkene intermediate is formed by a side reaction. Should the alkyl halides be bulky in nature, especially at the halogen end, there is a greater amount of alkene formed.
Methane cannot be synthesized by the Wurtz reaction, since at least two carbon atoms must occur as a part of an organic coupling reaction.
When tertiary alkyl halides are used, the Wurtz coupling process normally fails.
So, in Wurtz reaction, sodium reacts with alkyl halides in the presence of ether to form corresponding alkane. Tertiary halides having bulky groups undergo side reactions due to steric hindrance which results in an elimination reaction.
Hence, the given compound will have a very high tendency of undergoing elimination and hence will not undergo Wurtz reaction.
Note: Wurtz reaction is not preferred for preparation of alkane containing odd numbers of carbon atoms because in wurtz reaction the reactants are two different alkyl halides and due to which a mixture of alkanes is formed in the product. Also methane cannot be prepared using a wurtz reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




