
Why is vanillin achiral?
Answer
493.8k+ views
Hint: A molecule is said to be achiral when it has a superimposable mirror image. To identify a molecule as achiral it should not have a chiral centre, i.e. no carbon atom should have 4 different groups attached to it. As they are achiral they do not have a centre or plane of symmetry.
Complete Step By Step Answer:
To find out if Vanillin is achiral we will look into its molecular structure.
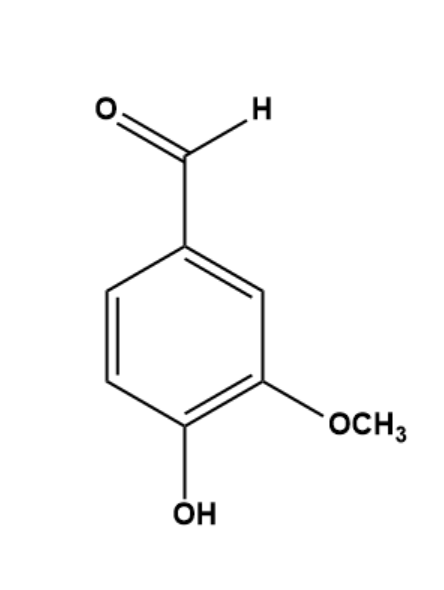
The structure of Vanillin shows the presence of an aldehyde group, an ether group, and a hydroxyl group. The IUPAC name of Vanillin is 4-hydroxy-3-methoxy benzaldehyde. This shows aldehyde is the group with the highest preference, followed by hydroxyl and then ether.
The Carbon atom attached to the Aldehyde group is attached to three other carbon atoms only. In the aromatic ring, no carbon atom is attached to 4 different groups forming a chiral centre except the methyl group in ether. Also, in the methyl group hydrogen is attached to 3 of the sides which does not make it chiral. And we established the fact that a chiral centre is important for a compound to be Chiral. Also, it has a mirror plane which means it is coplanar.
Therefore, Vanillin is achiral.
Note:
Superimposable mirror images are those which overlap each other when placed on top of each other. Always keep in mind non- superimposable images do not overlap and are the characteristic of enantiomers. Here Vanillin is a phenolic aldehyde since it contains a hydroxyl group at the para position.
Complete Step By Step Answer:
To find out if Vanillin is achiral we will look into its molecular structure.

The structure of Vanillin shows the presence of an aldehyde group, an ether group, and a hydroxyl group. The IUPAC name of Vanillin is 4-hydroxy-3-methoxy benzaldehyde. This shows aldehyde is the group with the highest preference, followed by hydroxyl and then ether.
The Carbon atom attached to the Aldehyde group is attached to three other carbon atoms only. In the aromatic ring, no carbon atom is attached to 4 different groups forming a chiral centre except the methyl group in ether. Also, in the methyl group hydrogen is attached to 3 of the sides which does not make it chiral. And we established the fact that a chiral centre is important for a compound to be Chiral. Also, it has a mirror plane which means it is coplanar.
Therefore, Vanillin is achiral.
Note:
Superimposable mirror images are those which overlap each other when placed on top of each other. Always keep in mind non- superimposable images do not overlap and are the characteristic of enantiomers. Here Vanillin is a phenolic aldehyde since it contains a hydroxyl group at the para position.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




