
Why is fructose hexose?
Answer
535.5k+ views
Hint: Fructose is a form of carbohydrate. Fructose is classified under carbohydrates as a monosaccharide. These are the simplest unit of carbohydrates.
Complete step-by-step answer:Fructose is known as a carbohydrate with the formula, ${{C}_{6}}{{H}_{12}}{{O}_{6}}$. It is a sugar due to its sweet taste. Fructose is an example of reducing sugar. Reducing sugars are those that contain free aldehydic, (CHO) or keto, (CO) group.
The structure of fructose contains a keto group along with a carbon chain. It contains a keto group on the second carbon atom. Along with the ketonic group, as its empirical formula suggests, fructose has a 6-carbon chain. It exists as dextrorotatory or levorotatory, D, and L that contain OH group on the right hand side of the second last carbon atom in D, while on the left in L configuration.
The D, and L fischer structures of fructose are as follows:
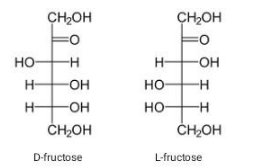
Due to the presence of a keto group and six carbon atoms, fructose is also called ketohexose.
Hence, fructose is a hexose due to the presence of 6-carbon atoms.
Additional information: Fructose and Glucose have the same formula ${{C}_{6}}{{H}_{12}}{{O}_{6}}$, but glucose is an aldohexose, while fructose is a ketohexose. They both are obtained on hydrolysis of a disaccharide, sucrose ${{C}_{12}}{{H}_{22}}{{O}_{11}}$.
Note: Another structure of fructose apart from chain structure is the open structure and the cyclic furanose structure. It also consists of two anomeric structures and exist as $\alpha $ and $\beta $, D-fructose.
Complete step-by-step answer:Fructose is known as a carbohydrate with the formula, ${{C}_{6}}{{H}_{12}}{{O}_{6}}$. It is a sugar due to its sweet taste. Fructose is an example of reducing sugar. Reducing sugars are those that contain free aldehydic, (CHO) or keto, (CO) group.
The structure of fructose contains a keto group along with a carbon chain. It contains a keto group on the second carbon atom. Along with the ketonic group, as its empirical formula suggests, fructose has a 6-carbon chain. It exists as dextrorotatory or levorotatory, D, and L that contain OH group on the right hand side of the second last carbon atom in D, while on the left in L configuration.
The D, and L fischer structures of fructose are as follows:

Due to the presence of a keto group and six carbon atoms, fructose is also called ketohexose.
Hence, fructose is a hexose due to the presence of 6-carbon atoms.
Additional information: Fructose and Glucose have the same formula ${{C}_{6}}{{H}_{12}}{{O}_{6}}$, but glucose is an aldohexose, while fructose is a ketohexose. They both are obtained on hydrolysis of a disaccharide, sucrose ${{C}_{12}}{{H}_{22}}{{O}_{11}}$.
Note: Another structure of fructose apart from chain structure is the open structure and the cyclic furanose structure. It also consists of two anomeric structures and exist as $\alpha $ and $\beta $, D-fructose.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




