
White phosphorus is stored in:
[A] In air
[B] Under water
[C] In kerosene
[D] Under $C{{S}_{2}}$
Answer
589.8k+ views
HINT: White phosphorus is the commonly found in elemental form of phosphorus. It is highly reactive in air. We store it in absence of air where it can be moist so that it does not react. It is soluble in ionic liquids and oil so we cannot use them for storing white phosphorus.
COMPLETE STEP BY STEP SOLUTION: We know that phosphorus is a group 15 element. phosphorus can exist in several allotropes. In elemental form phosphorus exists as ${{P}_{4}}$. However, its most common allotrope is red phosphorus which is a polymeric chain structure of ${{P}_{4}}$. Some allotropes like solid violet and black phosphorus are also known. In a gaseous state, phosphorus exists as di-phosphorus and atomic phosphorus.
Now, let us discuss about white phosphorus.
White phosphorus is the elemental form phosphorus and it is also known as tetra phosphorus. Each phosphorus atom is attached to another phosphorus atom with a single bond and the four atoms are arranged in a tetrahedral structure. We can draw the structure as-
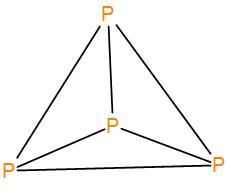
There are six P-P bonds here and bond length is around 225pm.
White phosphorus is a waxy solid and it is translucent. When we expose white phosphorus to light, it becomes yellow and thus it is also known as yellow phosphorus. It is highly flammable and burns with a green flame with oxygen. White phosphorus is highly reactive and ignites itself upon contact with air. For this reason, we cannot store white phosphorus in the air.
Now let us discuss where we can store white phosphorus.
In the first option we have air. We have discussed above that it is highly reactive and self-ignites when stored in air. So this option is incorrect.
Then we have water. To prevent its contact with air, we store white phosphorus in water. Therefore, this option is correct.
Then we have kerosene oil. White phosphorus is also soluble in oil so we cannot store it in kerosene oil either.
And lastly we have carbon disulphide. White phosphorus is also soluble in carbon disulphide which is an ionic liquid.
We can see from the above discussion that we can store white phosphorus in water.
Therefore, the correct answer is option [B] under water.
NOTE: White phosphorus is highly toxic and it can cause liver damage and phosphorus necrosis of jaw. Combustion of phosphorus gives phosphorus pentoxide which is a strong dehydrating agent as it is highly hygroscopic. It quickly absorbs moisture around it and forms phosphoric acid.
\[\begin{align}
& {{P}_{4}}+5{{O}_{2}}\to {{P}_{4}}{{O}_{10}} \\
& {{P}_{4}}{{O}_{10}}+6{{H}_{2}}O\to 4{{H}_{3}}P{{O}_{4}} \\
\end{align}\]
White phosphorus is used as a weapon due to its property and is immediately ignited upon contact with air.
COMPLETE STEP BY STEP SOLUTION: We know that phosphorus is a group 15 element. phosphorus can exist in several allotropes. In elemental form phosphorus exists as ${{P}_{4}}$. However, its most common allotrope is red phosphorus which is a polymeric chain structure of ${{P}_{4}}$. Some allotropes like solid violet and black phosphorus are also known. In a gaseous state, phosphorus exists as di-phosphorus and atomic phosphorus.
Now, let us discuss about white phosphorus.
White phosphorus is the elemental form phosphorus and it is also known as tetra phosphorus. Each phosphorus atom is attached to another phosphorus atom with a single bond and the four atoms are arranged in a tetrahedral structure. We can draw the structure as-

There are six P-P bonds here and bond length is around 225pm.
White phosphorus is a waxy solid and it is translucent. When we expose white phosphorus to light, it becomes yellow and thus it is also known as yellow phosphorus. It is highly flammable and burns with a green flame with oxygen. White phosphorus is highly reactive and ignites itself upon contact with air. For this reason, we cannot store white phosphorus in the air.
Now let us discuss where we can store white phosphorus.
In the first option we have air. We have discussed above that it is highly reactive and self-ignites when stored in air. So this option is incorrect.
Then we have water. To prevent its contact with air, we store white phosphorus in water. Therefore, this option is correct.
Then we have kerosene oil. White phosphorus is also soluble in oil so we cannot store it in kerosene oil either.
And lastly we have carbon disulphide. White phosphorus is also soluble in carbon disulphide which is an ionic liquid.
We can see from the above discussion that we can store white phosphorus in water.
Therefore, the correct answer is option [B] under water.
NOTE: White phosphorus is highly toxic and it can cause liver damage and phosphorus necrosis of jaw. Combustion of phosphorus gives phosphorus pentoxide which is a strong dehydrating agent as it is highly hygroscopic. It quickly absorbs moisture around it and forms phosphoric acid.
\[\begin{align}
& {{P}_{4}}+5{{O}_{2}}\to {{P}_{4}}{{O}_{10}} \\
& {{P}_{4}}{{O}_{10}}+6{{H}_{2}}O\to 4{{H}_{3}}P{{O}_{4}} \\
\end{align}\]
White phosphorus is used as a weapon due to its property and is immediately ignited upon contact with air.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




