
Which sublevel is filled just before 5f?
(A) 4f
(B) 7s
(C) 5d
(D) 6d
Answer
590.4k+ views
Hint: Read the Aufbau’s principle to obtain the answer for this question. You can also take a look at the periodic table to see which group is filled before the 5f orbital.
Complete step by step answer:
-According to Aufbau’s principle, ‘In the ground state of the atoms, the orbitals are filled with electrons in the order of their increasing energies.’
-Orbitals are filled in the order of increasing value of (n+l). For example, for 3d, n=3 and l=2, so, (n+l)=5 and for 4s, n=4 and l=0, so, (n+l)=4. As the value of (n+l) for 4s orbital is less than that of 3d orbital, 4s orbital is filled before 3d orbitals.
-Similarly, let us calculate (n+l) values for all the orbitals given in the question.
For 5f orbital, n=5 and l=3, so (n+l)=8
For 4f orbital, n=4 and l=3, so (n+l)=7
For 7s orbital, n=7 and l=0, so (n+l)=7
For 5d orbital, n=5 and l=2, so (n+l)=7
For 6d orbital, n=6 and l=2, so (n+l)=8
-If two orbitals have the same value of (n+l), then the orbital with lower value of n will be filled first. Now here, 4f has the least n-value so it will be filled first. Then comes, 5d and then 7s orbitals followed by 5f orbital and then 6d orbital.
-Aufbau’s diagram is shown below:
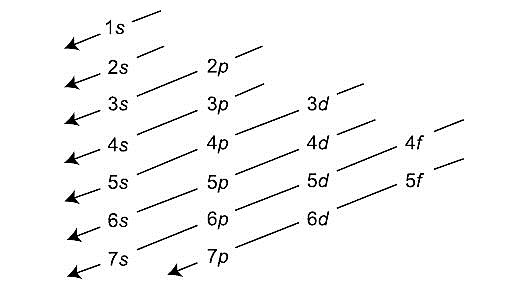
-Therefore, we can conclude that 7s-orbital is filled just before 5f-orbital.
So, the correct answer is “Option B”.
Note: Learn the Aufbau schematic diagram. Remember according to the rules, we need to check (n+l) values first and then if they are the same, then we go for an orbital having lower n-value.
Complete step by step answer:
-According to Aufbau’s principle, ‘In the ground state of the atoms, the orbitals are filled with electrons in the order of their increasing energies.’
-Orbitals are filled in the order of increasing value of (n+l). For example, for 3d, n=3 and l=2, so, (n+l)=5 and for 4s, n=4 and l=0, so, (n+l)=4. As the value of (n+l) for 4s orbital is less than that of 3d orbital, 4s orbital is filled before 3d orbitals.
-Similarly, let us calculate (n+l) values for all the orbitals given in the question.
For 5f orbital, n=5 and l=3, so (n+l)=8
For 4f orbital, n=4 and l=3, so (n+l)=7
For 7s orbital, n=7 and l=0, so (n+l)=7
For 5d orbital, n=5 and l=2, so (n+l)=7
For 6d orbital, n=6 and l=2, so (n+l)=8
-If two orbitals have the same value of (n+l), then the orbital with lower value of n will be filled first. Now here, 4f has the least n-value so it will be filled first. Then comes, 5d and then 7s orbitals followed by 5f orbital and then 6d orbital.
-Aufbau’s diagram is shown below:

-Therefore, we can conclude that 7s-orbital is filled just before 5f-orbital.
So, the correct answer is “Option B”.
Note: Learn the Aufbau schematic diagram. Remember according to the rules, we need to check (n+l) values first and then if they are the same, then we go for an orbital having lower n-value.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




