
Which statement about aspirin is not true?
A. Aspirin belongs to narcotic analgesics.
B. It is effective in relieving pain.
C. It has anti-blood clotting action
D. It is a neurologically active drug.
Answer
572.1k+ views
Hint:Aspirin is chemically known as acetylsalicylic acid. The chemical formula of aspirin is ${{{C}}_9}{{{H}}_8}{{{O}}_4}$ It is included in the group of drugs. When salicylic acid is reacted with acetic anhydride in the presence of sulfuric acid, aspirin and acetic acid are obtained.
Complete answer:
Aspirin is an ester. It is a combination of alcohol, ester and acid. It comes under the group of salicylate drugs. It is a weak acid. It is considered as a medicine.
During the preparation of aspirin, salicylic acid and aspirin causes irritation to the skin. Also acetic anhydride and sulfuric acid can cause burns.
The preparation of aspirin is given in the reaction given below:
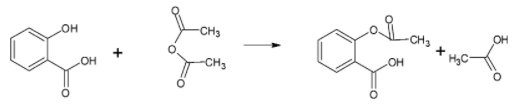
Since the compound contains one acetyl group and an acid group, it is acidic in nature.
Heart attacks, strokes and chest pains can be treated using aspirin. Thus we can say that it can be used as a painkiller. Prostaglandins stimulates inflammation and causes pain. But aspirin stops the production of prostaglandins. It also prevents the platelet coagulation. Thus it has anti-blood clotting action. So it is used in treating heart attacks.
Thus aspirin does not belong to narcotic analgesics.
Hence, the correct option is A.
Note:
Aspirin can reduce risk of getting cancer. It is used in serious conditions. Thus it must not be given to children having fever. It may cause Reye’s syndrome in children. Moreover, alcohol usage during medication may cause bleeding in the stomach.
Complete answer:
Aspirin is an ester. It is a combination of alcohol, ester and acid. It comes under the group of salicylate drugs. It is a weak acid. It is considered as a medicine.
During the preparation of aspirin, salicylic acid and aspirin causes irritation to the skin. Also acetic anhydride and sulfuric acid can cause burns.
The preparation of aspirin is given in the reaction given below:

Since the compound contains one acetyl group and an acid group, it is acidic in nature.
Heart attacks, strokes and chest pains can be treated using aspirin. Thus we can say that it can be used as a painkiller. Prostaglandins stimulates inflammation and causes pain. But aspirin stops the production of prostaglandins. It also prevents the platelet coagulation. Thus it has anti-blood clotting action. So it is used in treating heart attacks.
Thus aspirin does not belong to narcotic analgesics.
Hence, the correct option is A.
Note:
Aspirin can reduce risk of getting cancer. It is used in serious conditions. Thus it must not be given to children having fever. It may cause Reye’s syndrome in children. Moreover, alcohol usage during medication may cause bleeding in the stomach.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




