
Which side products are formed in the aldol condensation of dibenzalacetone?
Answer
497.7k+ views
Hint: An aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form $ \beta - $ -hydroxy aldehyde or $ \beta - $ hydroxy ketone, followed by dehydration to give a conjugated enone. The first part of this reaction is an aldol reaction, the second part a dehydration and elimination reaction.
Complete Step By Step Answer:
Dibenzalacetone is readily prepared by condensation of acetone with two equivalents of benzaldehyde. The aldehyde carbonyl is more reactive than that of the ketone and therefore reacts rapidly with the anion of the ketone to give a $ \beta - $ hydroxy ketone, which easily undergoes base catalyzed dehydration. Depending on the relative quantities of the reactant the reaction can give either mono- or dibenzalacetone.
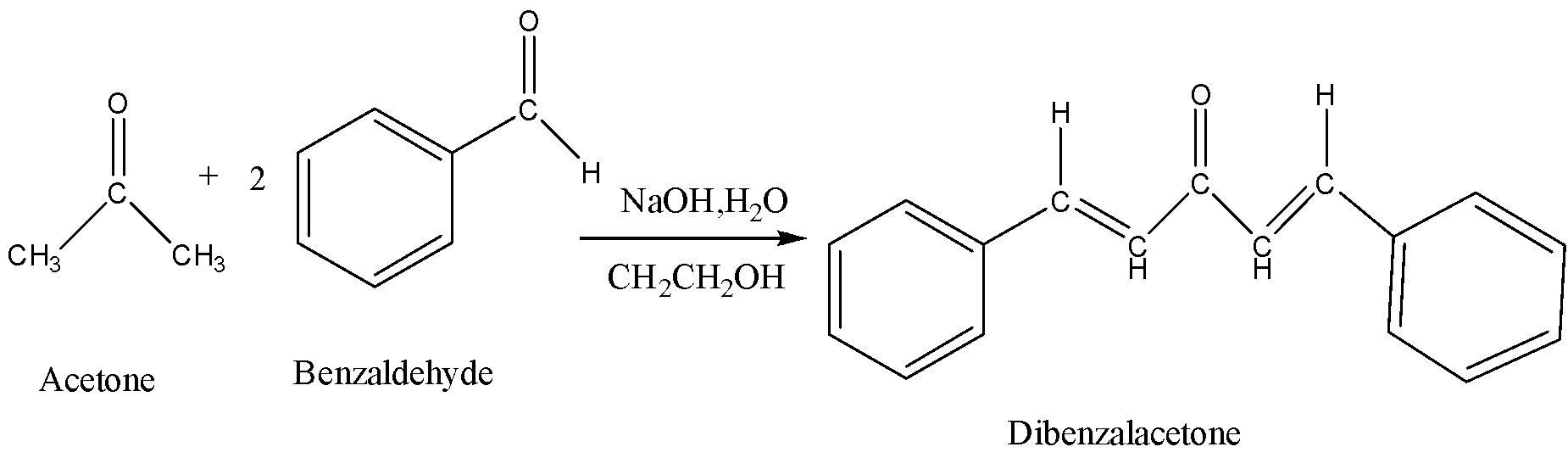
In mixed aldol condensation, one molecule of aldehyde reacts with the other molecule of aldehyde. The reaction between acetone and two molecules of benzaldehyde to form dibenzalacetone is shown below. Therefore, the side product formed in the synthesis of dibenzalacetone is $ {H_2}O $ .
Additional Information:
A crossed aldol condensation is a result of two dissimilar carbonyl compounds containing $ \alpha - $ hydrogen undergoing aldol condensation. Ordinarily, this leads to four possible products as either carbonyl compound can act as the nucleophile and self-condensation is possible, which makes a synthetically useless mixture. However, this problem can be avoided if one of the compounds does not contain an $ \alpha - $ hydrogen, rendering it non-enolizable.
Note:
Dibenzalacetone is also referred to as dibenzylideneacetone and dba. It's an organic compound with the formula $ {C_{17}}{H_{14}}O $ . It's used as a component in sunscreens, and some industrial organometallic compounds because it bonds to metals and helps form a stable chemical structure.
Complete Step By Step Answer:
Dibenzalacetone is readily prepared by condensation of acetone with two equivalents of benzaldehyde. The aldehyde carbonyl is more reactive than that of the ketone and therefore reacts rapidly with the anion of the ketone to give a $ \beta - $ hydroxy ketone, which easily undergoes base catalyzed dehydration. Depending on the relative quantities of the reactant the reaction can give either mono- or dibenzalacetone.

In mixed aldol condensation, one molecule of aldehyde reacts with the other molecule of aldehyde. The reaction between acetone and two molecules of benzaldehyde to form dibenzalacetone is shown below. Therefore, the side product formed in the synthesis of dibenzalacetone is $ {H_2}O $ .
Additional Information:
A crossed aldol condensation is a result of two dissimilar carbonyl compounds containing $ \alpha - $ hydrogen undergoing aldol condensation. Ordinarily, this leads to four possible products as either carbonyl compound can act as the nucleophile and self-condensation is possible, which makes a synthetically useless mixture. However, this problem can be avoided if one of the compounds does not contain an $ \alpha - $ hydrogen, rendering it non-enolizable.
Note:
Dibenzalacetone is also referred to as dibenzylideneacetone and dba. It's an organic compound with the formula $ {C_{17}}{H_{14}}O $ . It's used as a component in sunscreens, and some industrial organometallic compounds because it bonds to metals and helps form a stable chemical structure.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




