
Which reagent will convert $ RCOR' $ group into $ RR'C\left( {{C_6}{H_5}} \right)OH $ ?
Answer
522k+ views
Hint : $ RCOR' $ is a ketone group. For solving this particular reaction you have to look into all the reactions a ketone undergoes. Another thing to note is that $ RR'C\left( {{C_6}{H_5}} \right)OH $ is our product which has $ {C_6}{H_5} $ (Benzene) and $ - OH $ in it which means either the reactant or reagent has $ {C_6}{H_5} $ and $ - OH $ group in them. Our reactant does not have these groups so the reactant that we get should have then or end up giving these groups to the product. Taking all this into account the question can be solved accordingly.
Complete Step By Step Answer:
When we look at our reagent $ RCOR' $ , it is a ketone molecule where $ R $ and $ R' $ are two different alkyl groups. It undergoes many different types of reactions. So to solve this particular question we will take into account the product that is given, which is $ RR'C\left( {{C_6}{H_5}} \right)OH $ .
As we can see in the product that we have benzene as well as an alcohol group attached to it along with the two $ R $ and $ R' $ groups present in the reactant as well. So during this reaction $ > CO $ is involved in the reaction and changing into the product.
$ RCOR'\xrightarrow{?}RR'C\left( {{C_6}{H_5}} \right)OH $ So this is our reaction and we have to find what is ‘?’
The best reagent that we find suitable for this reaction is Grignard reagent $ RMgX + {H_2}O $ where $ R $ in our case will be $ {C_6}{H_5} $ group, because our product has $ {C_6}{H_5} $ . So the reaction of $ RCOR' $ with Grignard reagent will be as follows:
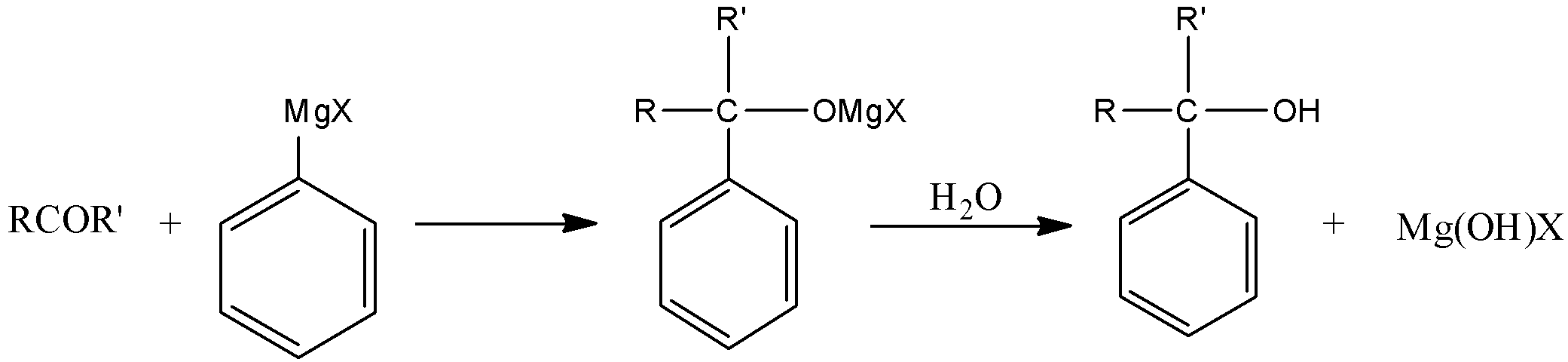
Where $ R $ and $ R' $ are two different alkyl groups and $ X $ is any halogen.
Therefore the reagent that will convert $ RCOR' $ to $ RR'C\left( {{C_6}{H_5}} \right)OH $ is Grignard reagent (Alkyl, Vinyl and aryl magnesium halides) and in our case it is phenyl magnesium halide ( $ {C_6}{H_5}MgX $ ).
Note :
For solving such questions analyzing the reactant and the product that it forms, carefully is very important. As one reactant undergoes many different reactions. For this particular problem, the reagent we get is Grignard reagent $ {C_6}{H_5}MgX $ , which is phenyl substituted. This reaction can also take place with alkyl magnesium halide $ RMgX $ but then it will yield an alkyl product, not the product that we require in this particular reaction.
Complete Step By Step Answer:
When we look at our reagent $ RCOR' $ , it is a ketone molecule where $ R $ and $ R' $ are two different alkyl groups. It undergoes many different types of reactions. So to solve this particular question we will take into account the product that is given, which is $ RR'C\left( {{C_6}{H_5}} \right)OH $ .
As we can see in the product that we have benzene as well as an alcohol group attached to it along with the two $ R $ and $ R' $ groups present in the reactant as well. So during this reaction $ > CO $ is involved in the reaction and changing into the product.
$ RCOR'\xrightarrow{?}RR'C\left( {{C_6}{H_5}} \right)OH $ So this is our reaction and we have to find what is ‘?’
The best reagent that we find suitable for this reaction is Grignard reagent $ RMgX + {H_2}O $ where $ R $ in our case will be $ {C_6}{H_5} $ group, because our product has $ {C_6}{H_5} $ . So the reaction of $ RCOR' $ with Grignard reagent will be as follows:

Where $ R $ and $ R' $ are two different alkyl groups and $ X $ is any halogen.
Therefore the reagent that will convert $ RCOR' $ to $ RR'C\left( {{C_6}{H_5}} \right)OH $ is Grignard reagent (Alkyl, Vinyl and aryl magnesium halides) and in our case it is phenyl magnesium halide ( $ {C_6}{H_5}MgX $ ).
Note :
For solving such questions analyzing the reactant and the product that it forms, carefully is very important. As one reactant undergoes many different reactions. For this particular problem, the reagent we get is Grignard reagent $ {C_6}{H_5}MgX $ , which is phenyl substituted. This reaction can also take place with alkyl magnesium halide $ RMgX $ but then it will yield an alkyl product, not the product that we require in this particular reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




