
Which reagent converts propene to 1-propanol?
(A) \[{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}\]
(B) \[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\]
(C) \[Hg{{(OAc)}_{2}},NaB{{H}_{4}}/{{H}_{2}}O\]
(D) $Aq. KOH$
Answer
588k+ views
Hint: The structure of propene is as follows.
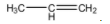
The structure of 1-Propanol is as follows.
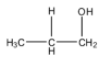
In the structure of 1-propanol, the alcohol group is present on the terminal carbon atom.
Normal reagents react with propene and form 2-propanol as the product.
We need selective chemicals to get 1-propanol from propene.
Complete step by step solution:
-In the question, it is asked which chemical is going to convert propene into 1-propanol.
-Coming to given option, option A \[{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}\].
-The reaction of propene with \[{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}\]is as follows.
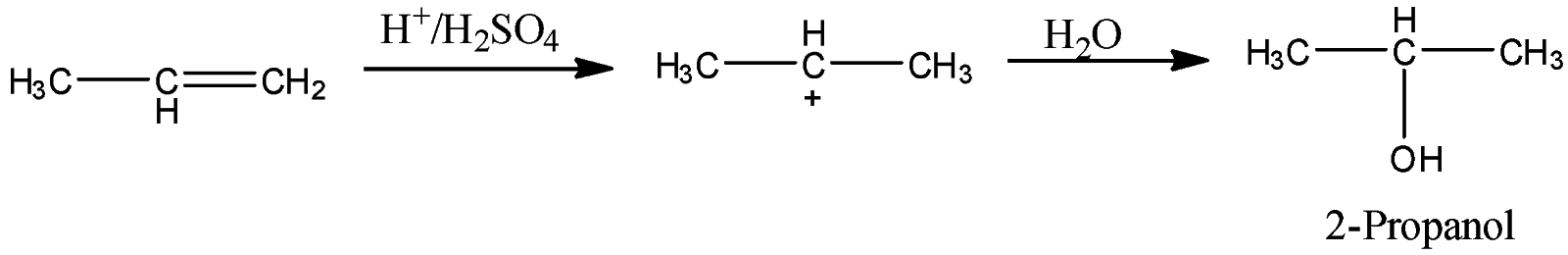
-Propene reacts with \[{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}\]and forms a product called 2-propanol. So, Option A is wrong.
-Coming to option B, \[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\].
- The reaction of propene with \[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\]is as follows.
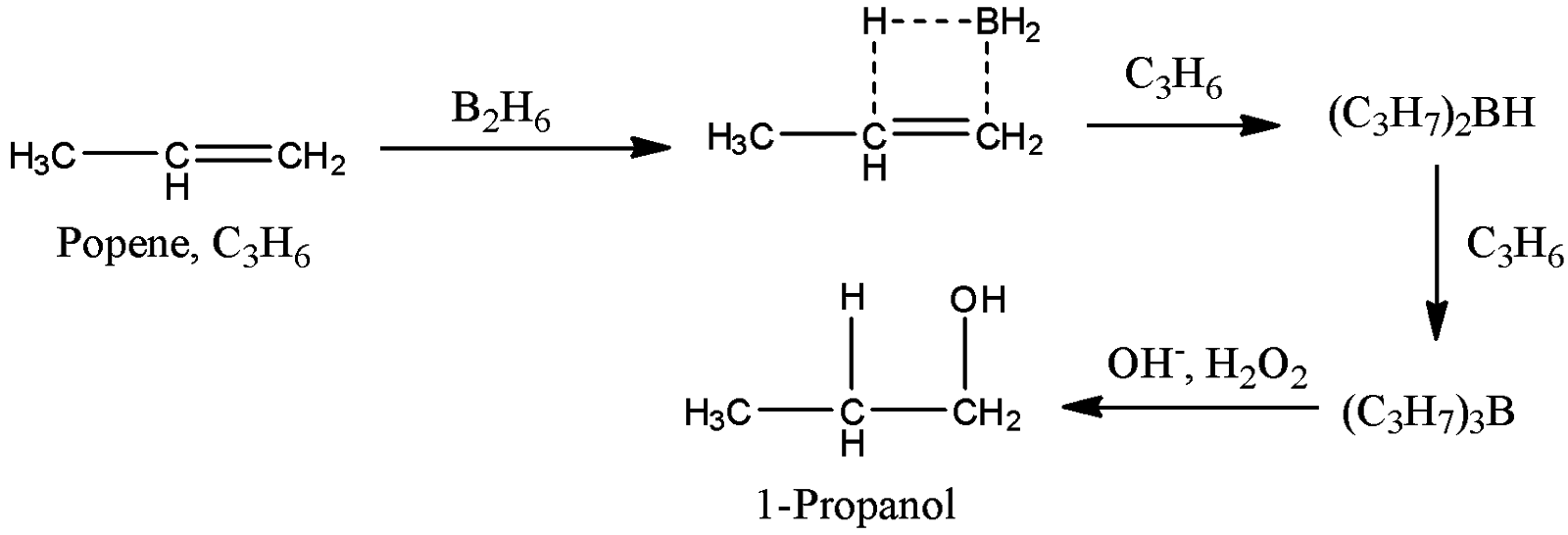
- Propene reacts with \[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\]and forms 1-propanol as a product. Therefore option B is correct.
-Coming to option C, \[Hg{{(OAc)}_{2}},NaB{{H}_{4}}/{{H}_{2}}O\]
-The reaction of propene with \[Hg{{(OAc)}_{2}},NaB{{H}_{4}}/{{H}_{2}}O\] is as follows.
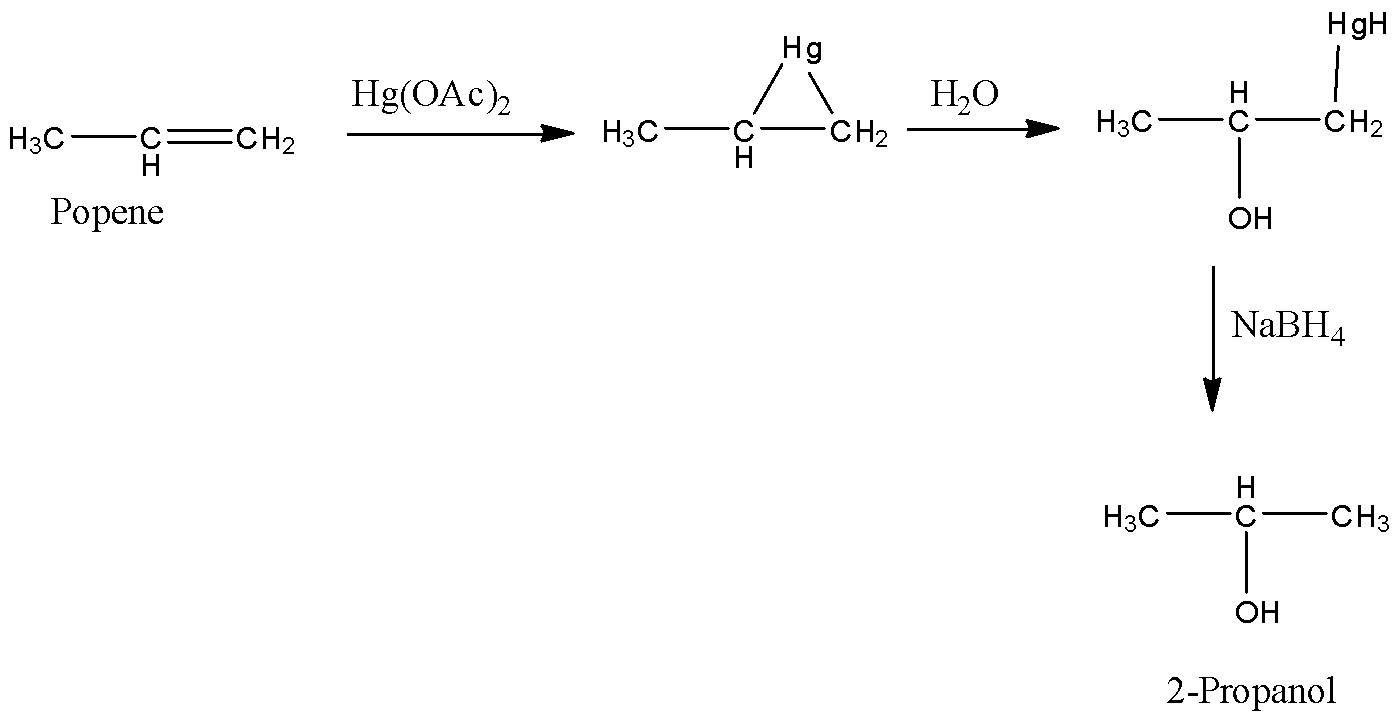
- Propene reacts with\[Hg{{(OAc)}_{2}},NaB{{H}_{4}}/{{H}_{2}}O\] and forms a product called 2-propanol. So, Option C is wrong.
- Coming to option D, Aq. KOH.
- The reaction of propene with Aq. KOH is as follows.
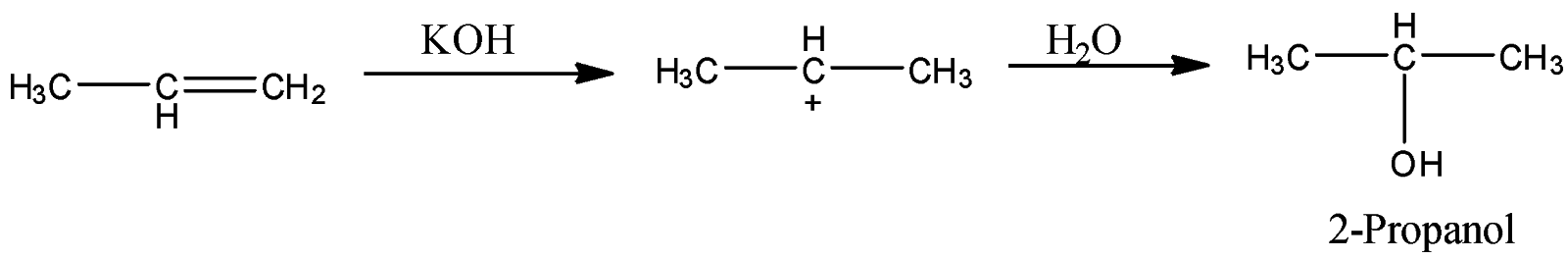
- Propene reacts with Aq. KOH and forms a product called 2-propanol. So, Option D is also wrong.
-Therefore reagent\[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\] converts propene to 1-propanol.
So, the correct option is B.
Note: The reaction of propene with \[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\]gives 1-propanol as the product. This reaction is called hydroboration. Hydroboration gives terminal alcohols as the product when reacts with an alkene. Hydroboration is a selective reaction to prepare terminal alcohols from alkenes.
The structure of 1-Propanol is as follows.

In the structure of 1-propanol, the alcohol group is present on the terminal carbon atom.
Normal reagents react with propene and form 2-propanol as the product.
We need selective chemicals to get 1-propanol from propene.
Complete step by step solution:
-In the question, it is asked which chemical is going to convert propene into 1-propanol.
-Coming to given option, option A \[{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}\].
-The reaction of propene with \[{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}\]is as follows.

-Propene reacts with \[{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}\]and forms a product called 2-propanol. So, Option A is wrong.
-Coming to option B, \[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\].
- The reaction of propene with \[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\]is as follows.

- Propene reacts with \[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\]and forms 1-propanol as a product. Therefore option B is correct.
-Coming to option C, \[Hg{{(OAc)}_{2}},NaB{{H}_{4}}/{{H}_{2}}O\]
-The reaction of propene with \[Hg{{(OAc)}_{2}},NaB{{H}_{4}}/{{H}_{2}}O\] is as follows.

- Propene reacts with\[Hg{{(OAc)}_{2}},NaB{{H}_{4}}/{{H}_{2}}O\] and forms a product called 2-propanol. So, Option C is wrong.
- Coming to option D, Aq. KOH.
- The reaction of propene with Aq. KOH is as follows.

- Propene reacts with Aq. KOH and forms a product called 2-propanol. So, Option D is also wrong.
-Therefore reagent\[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\] converts propene to 1-propanol.
So, the correct option is B.
Note: The reaction of propene with \[{{B}_{2}}{{H}_{6}},{{H}_{2}}{{O}_{2}},OH\]gives 1-propanol as the product. This reaction is called hydroboration. Hydroboration gives terminal alcohols as the product when reacts with an alkene. Hydroboration is a selective reaction to prepare terminal alcohols from alkenes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




