
Which reaction below is correct?
(A)
(B)
(C)
(D)




Answer
588.3k+ views
Hint: Oxymercuration-Demercuration reaction will do Markovnikov type of addition across the alkene double bond while Hydroboration-Oxidation will do Anti-markovnikov type of addition across the double bond. Osmium tetroxide will always give addition in a syn manner.
Complete step by step answer:
All the reaction given here is on the same compound and every reagent does addition to the alkene double bond. Let’s see which one of them is true one by one.
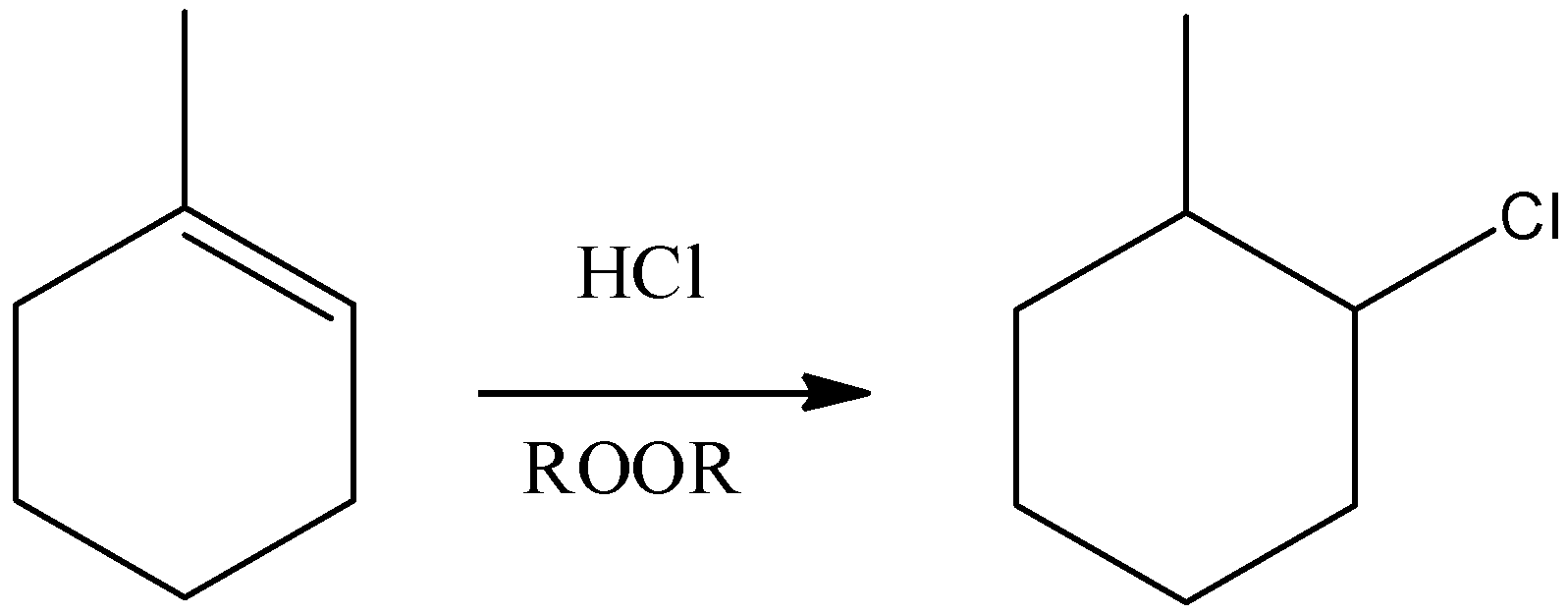
A) Here, we can see that HCl and a peroxide is allowed to react with a compound that has alkene functional group, addition over the double bond will occur. But this addition will be a markovnikov type addition that means the nucleophile will form a bond with the carbon that has the least number of hydrogen atoms.
- Remember that in presence of peroxides, only HBr gives Anti-markovnikov addition, HCl and HI cannot give that type of addition. So, this reaction will be as shown below as shown in the option. So, this reaction is correct.
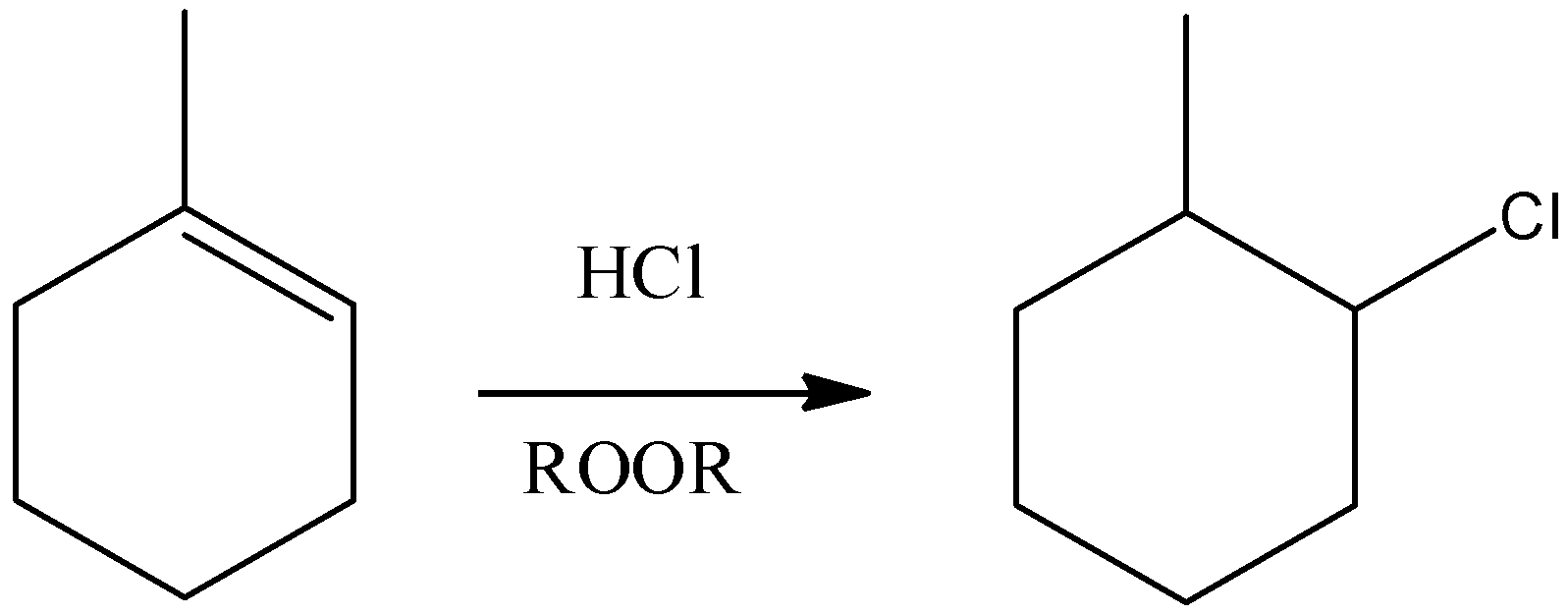
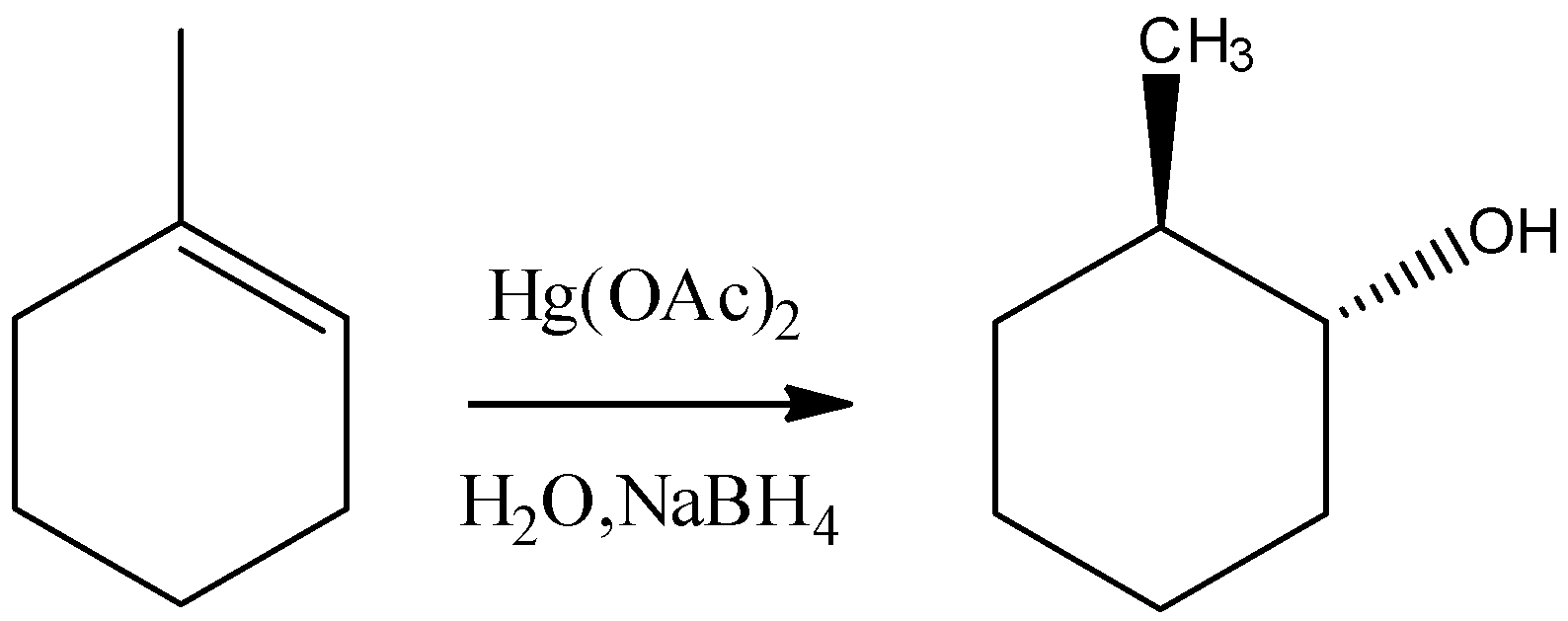
B) It is an Oxymercuration-Demercuration reaction. $Hg{(OAc)_2}$ reacts with alkenes which is called oxymercuration in which –HgOAc and –OH group gets attached across the double bond. The –OH group gets attached to the carbon having less number of hydrogens, hence it is a markovnikov type of addition. Moreover, the addition will be a syn addition. So, the final product will be:
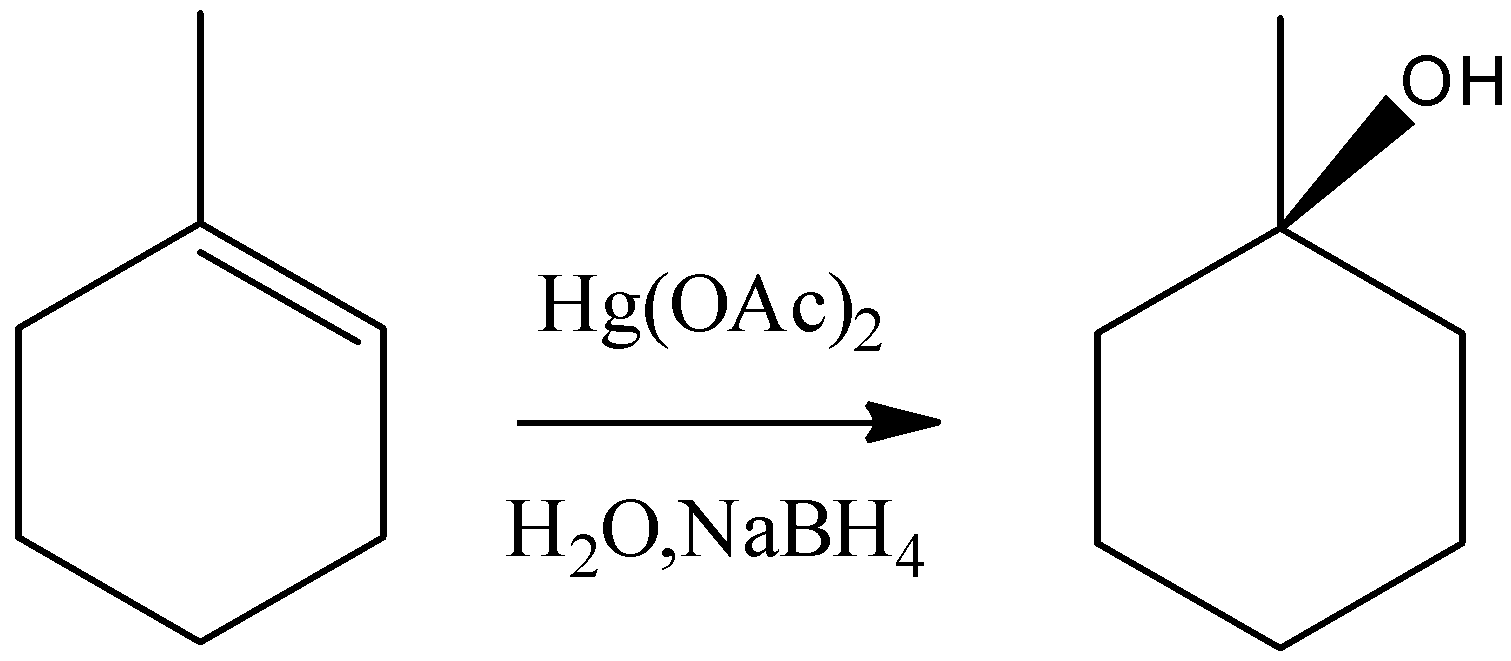
So, the reaction given in the option is not true.
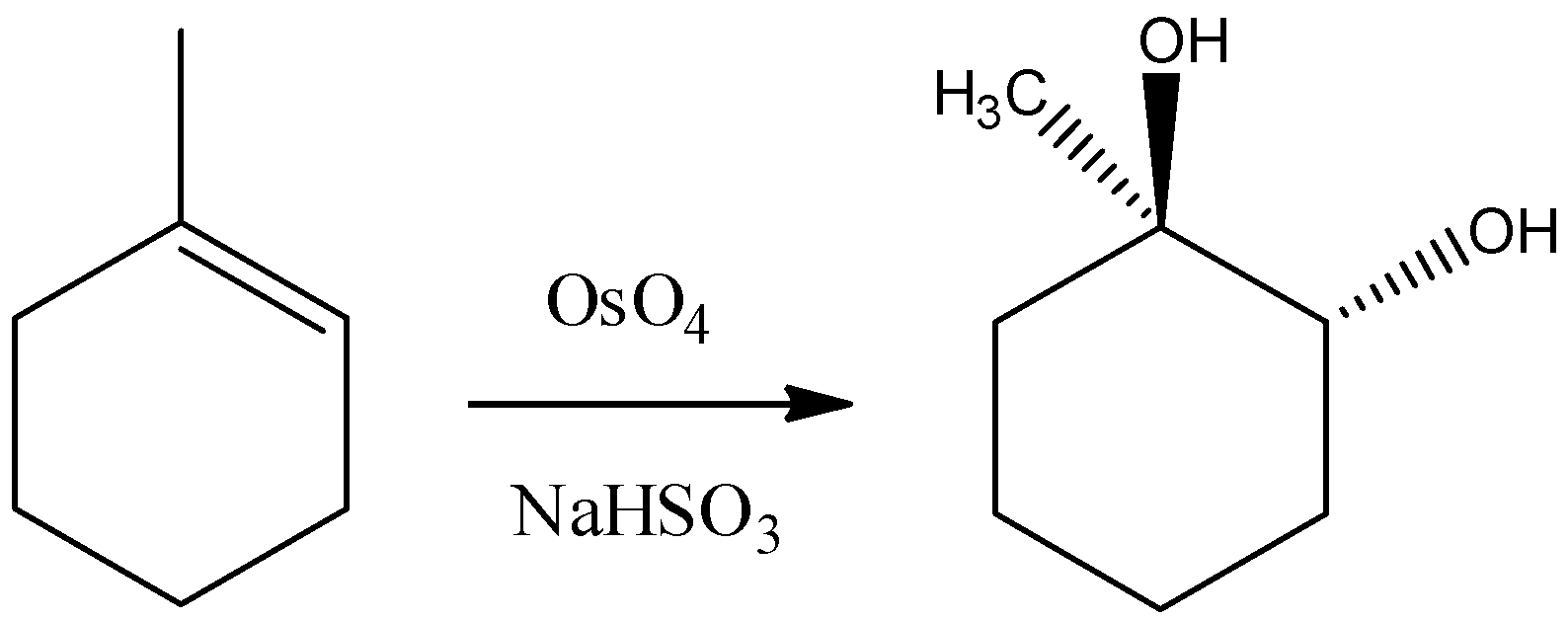
C) Osmium Tetroxide has four oxygen atoms bonded with central metal atom osmium. So, it is known for addition of hydroxyl groups at the alkene double bond. But remember that it follows a concerted pericyclic type mechanism and so that it will add –OH group in a syn manner. Hence we can say that the reaction would be as follows.
Thus, the reaction given in the option is incorrect.
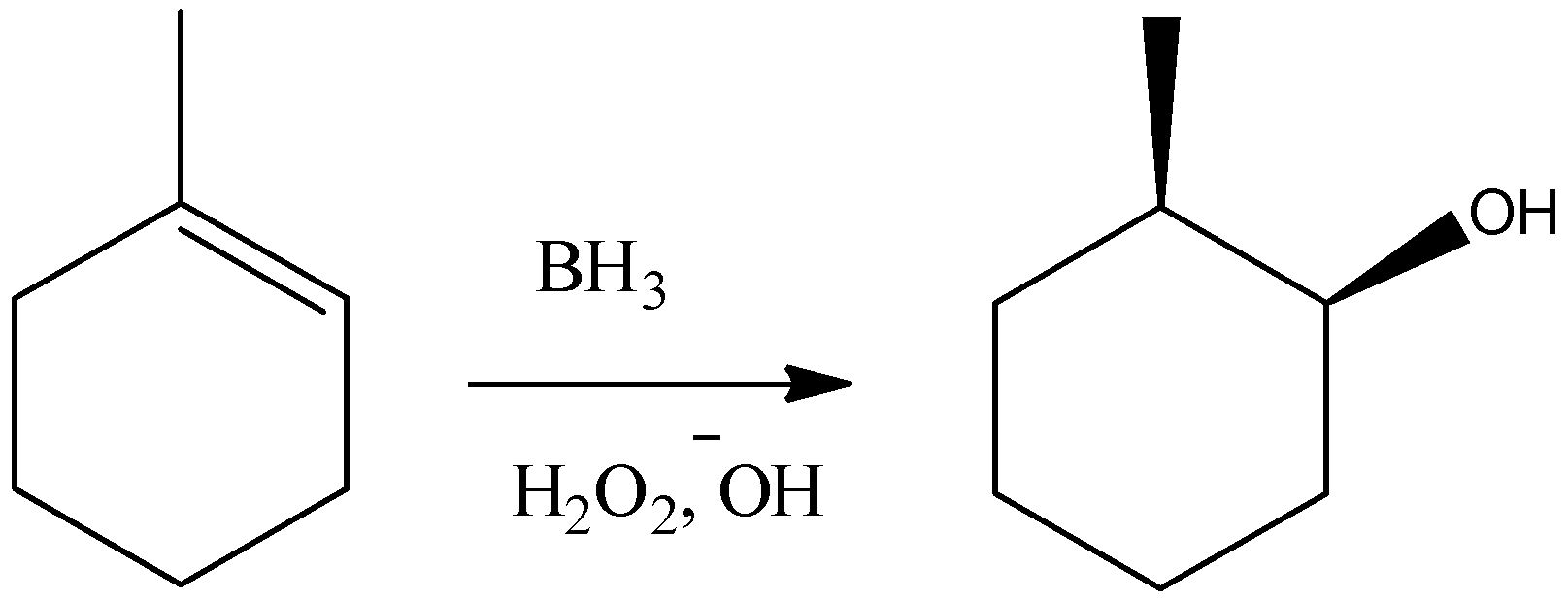
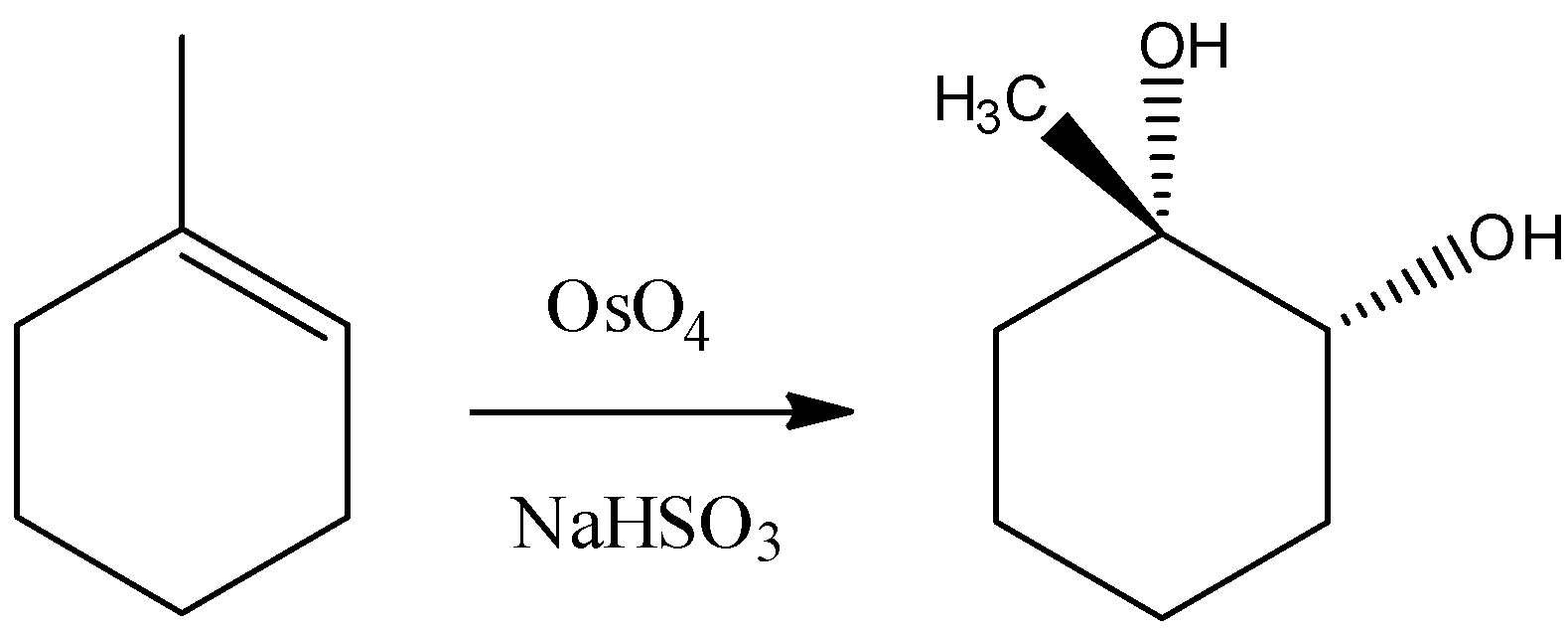
D) It is a Hydroboration-Oxidation reaction. It will add hydroxyl group and hydrogen atom over the alkene double bond in a syn manner. But as it follows a free radical mechanism, this reaction will do an anti-markovnikov type of addition over the double bond. So, we can write the reaction as:
So, the reaction given in the option is not correct.
So, the correct answer is “Option A”.
Note: Remember that even in presence of Alkyl peroxides, only HBr gives anti-markovnikov type of addition. Oxymercuration-Demercuration reaction always does syn and markovnikov type of addition.
Complete step by step answer:
All the reaction given here is on the same compound and every reagent does addition to the alkene double bond. Let’s see which one of them is true one by one.
A) Here, we can see that HCl and a peroxide is allowed to react with a compound that has alkene functional group, addition over the double bond will occur. But this addition will be a markovnikov type addition that means the nucleophile will form a bond with the carbon that has the least number of hydrogen atoms.
- Remember that in presence of peroxides, only HBr gives Anti-markovnikov addition, HCl and HI cannot give that type of addition. So, this reaction will be as shown below as shown in the option. So, this reaction is correct.

B) It is an Oxymercuration-Demercuration reaction. $Hg{(OAc)_2}$ reacts with alkenes which is called oxymercuration in which –HgOAc and –OH group gets attached across the double bond. The –OH group gets attached to the carbon having less number of hydrogens, hence it is a markovnikov type of addition. Moreover, the addition will be a syn addition. So, the final product will be:
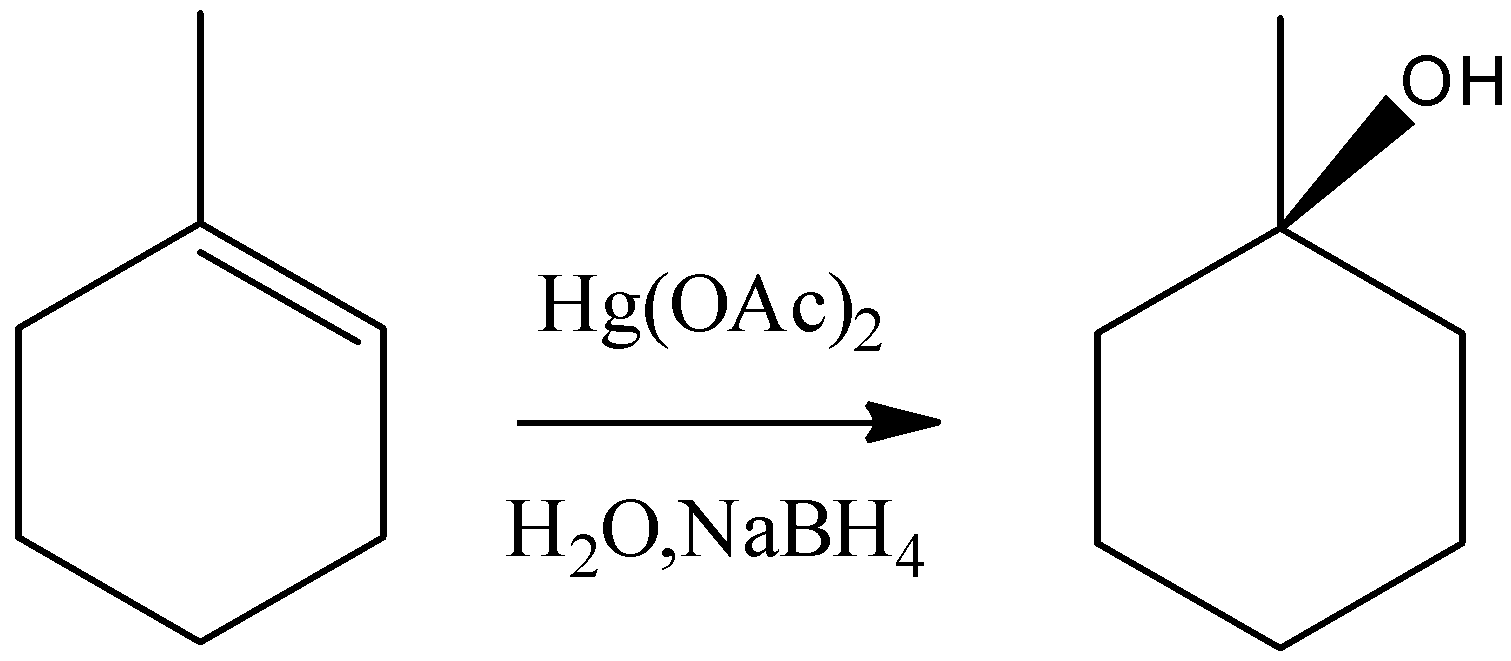
So, the reaction given in the option is not true.
C) Osmium Tetroxide has four oxygen atoms bonded with central metal atom osmium. So, it is known for addition of hydroxyl groups at the alkene double bond. But remember that it follows a concerted pericyclic type mechanism and so that it will add –OH group in a syn manner. Hence we can say that the reaction would be as follows.

Thus, the reaction given in the option is incorrect.
D) It is a Hydroboration-Oxidation reaction. It will add hydroxyl group and hydrogen atom over the alkene double bond in a syn manner. But as it follows a free radical mechanism, this reaction will do an anti-markovnikov type of addition over the double bond. So, we can write the reaction as:

So, the reaction given in the option is not correct.
So, the correct answer is “Option A”.
Note: Remember that even in presence of Alkyl peroxides, only HBr gives anti-markovnikov type of addition. Oxymercuration-Demercuration reaction always does syn and markovnikov type of addition.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




