
Which one of the following statements regarding Henry’s law is not correct?
A. The value of ${{K}_{H}}$ increases with the function of the nature of the gas
B. Higher the value of ${{K}_{H}}$at a given pressure, higher is the solubility of the gas in the liquids
C. The partial of the gas in vapour phase is proportional to the mole fraction of the gas in the solution.
D. Different gases have different ${{K}_{H}}$ (Henry’s law constant) values at the same temperature.
Answer
591.6k+ views
Hint: Henry’s law states that the amount of the dissolved gas in a liquid is found to be proportional to its partial pressure above the liquid. Mathematically Henry’s law can be written as: $P={{k}_{H}}.C$
Where, P is the partial pressure of gas, ${{k}_{H}}$is the proportionality constant, and C is the concentration of gas
Complete answer:
- As we know that $P={{k}_{H}}.C$. Partial pressure of gas is directly proportional to the proportionality constant and to the concentration of gas
- Statement (A) is correct, because as the value of ${{K}_{H}}$ increases with the function of the nature of the gas - this statement is correct because ${{K}_{H}}$ mainly depends on three things: nature of gas, nature of solvent, temperature and pressure. So, as the temperature increases the value of ${{K}_{H}}$also increases.
- Statement (B) is wrong because in the equation $P={{k}_{H}}.C$, we can see that pressure of gas is directly proportional to the concentration, and if proportionality constant is high then concentration will be low according to the equation. The solubility of the gas in the liquids will be low. And hence, this statement is wrong.
- Statement (C) is correct because if we see the equation of partial of the gas :${{P}_{A}}={{P}_{A}}^{\circ }.{{x}_{A}}$
Where,${{P}_{A}}$ is the partial pressure of gas in vapour phase, ${{P}_{A}}^{\circ }$ is the vapour pressure of pure component, ${{x}_{A}}$ is the mole fraction.
- Statement (D) is correct. Different gases have different ${{K}_{H}}$ (Henry’s law constant) values at the same temperature- If we compare three gases(oxygen, nitrogen and helium) then there solubility versus pressure graph will be like this:
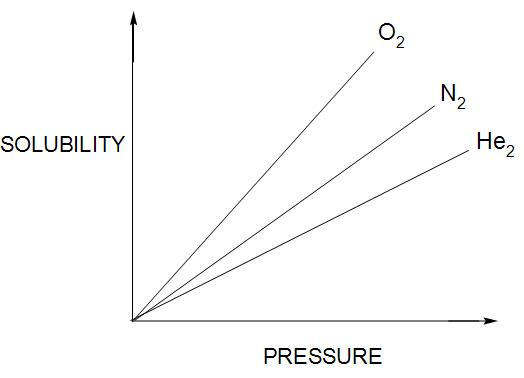
So, here we can see that pressure and solubility differs for each gas, hence, ${{K}_{H}}$will also differ.
Hence, we can say that the statement(B) is wrong.
Note:
- Henry’s law has many applications like in production of the carbonated beverages, in deep sea diving etc.
- This law is applicable only when the molecules of the system are present in a state of equilibrium.
- It is not applicable when gases are placed in high pressures.
Where, P is the partial pressure of gas, ${{k}_{H}}$is the proportionality constant, and C is the concentration of gas
Complete answer:
- As we know that $P={{k}_{H}}.C$. Partial pressure of gas is directly proportional to the proportionality constant and to the concentration of gas
- Statement (A) is correct, because as the value of ${{K}_{H}}$ increases with the function of the nature of the gas - this statement is correct because ${{K}_{H}}$ mainly depends on three things: nature of gas, nature of solvent, temperature and pressure. So, as the temperature increases the value of ${{K}_{H}}$also increases.
- Statement (B) is wrong because in the equation $P={{k}_{H}}.C$, we can see that pressure of gas is directly proportional to the concentration, and if proportionality constant is high then concentration will be low according to the equation. The solubility of the gas in the liquids will be low. And hence, this statement is wrong.
- Statement (C) is correct because if we see the equation of partial of the gas :${{P}_{A}}={{P}_{A}}^{\circ }.{{x}_{A}}$
Where,${{P}_{A}}$ is the partial pressure of gas in vapour phase, ${{P}_{A}}^{\circ }$ is the vapour pressure of pure component, ${{x}_{A}}$ is the mole fraction.
- Statement (D) is correct. Different gases have different ${{K}_{H}}$ (Henry’s law constant) values at the same temperature- If we compare three gases(oxygen, nitrogen and helium) then there solubility versus pressure graph will be like this:

So, here we can see that pressure and solubility differs for each gas, hence, ${{K}_{H}}$will also differ.
Hence, we can say that the statement(B) is wrong.
Note:
- Henry’s law has many applications like in production of the carbonated beverages, in deep sea diving etc.
- This law is applicable only when the molecules of the system are present in a state of equilibrium.
- It is not applicable when gases are placed in high pressures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




