
Which one of the following statements is correct about the given compound?
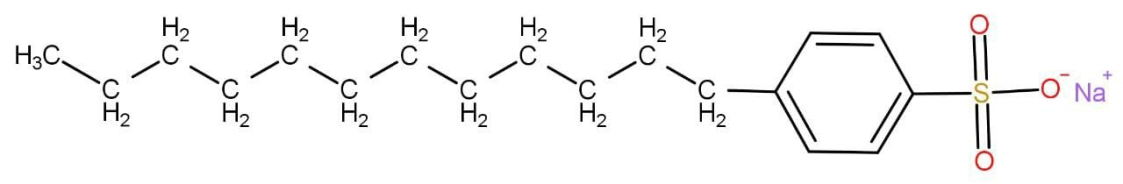
A.An anionic surfactant
B.A soap
C.A non ionic surfactant
D.A cationic surfactant

Answer
578.1k+ views
Hint: Since the positive and negative ions are present that means it’s an ionic compound. The soaps are sodium salts of fatty acid and surfactants are the organic compounds.
Complete step by step answer:
Surfactants are the compound that reduces the surface tension. The surfactant comprises two words that are surface active agents. There two parts in a surfactant are the head part and the tail part. The tail of most of the surfactants are very similar and consist of a hydrocarbon chain which can be aromatic or linear or branched.
Fluoro surfactants have fluoro-carbon chains and siloxane surfactants have siloxane groups.
Anionic surfactants as the name suggest contain anionic functional group that is a negatively charged functional group such as sulphate, sulphonate and phosphate etc. The one given in question is also an anionic surfactant.
Non ionic surfactants have covalent bonds. Usually the oxygen is attached with some hydrophilic group because the oxygen part will be soluble in water.
A soap is a long chain of sodium or potassium salt of fatty acid that is carboxylic acid. There is no carboxylic acid group in the molecule given and hence it is not soap.
Hence the correct option is A.
Note:
A hydrophilic part is that which is water loving, that is it gets dissolved in water. Hydro means water and philic means loving. A hydrophobic part is that which is water repelling, the phobic means repulsion. It does not dissolve in water but is soluble in oil. The surfactants are non toxic in nature.
Complete step by step answer:
Surfactants are the compound that reduces the surface tension. The surfactant comprises two words that are surface active agents. There two parts in a surfactant are the head part and the tail part. The tail of most of the surfactants are very similar and consist of a hydrocarbon chain which can be aromatic or linear or branched.
Fluoro surfactants have fluoro-carbon chains and siloxane surfactants have siloxane groups.
Anionic surfactants as the name suggest contain anionic functional group that is a negatively charged functional group such as sulphate, sulphonate and phosphate etc. The one given in question is also an anionic surfactant.
Non ionic surfactants have covalent bonds. Usually the oxygen is attached with some hydrophilic group because the oxygen part will be soluble in water.
A soap is a long chain of sodium or potassium salt of fatty acid that is carboxylic acid. There is no carboxylic acid group in the molecule given and hence it is not soap.
Hence the correct option is A.
Note:
A hydrophilic part is that which is water loving, that is it gets dissolved in water. Hydro means water and philic means loving. A hydrophobic part is that which is water repelling, the phobic means repulsion. It does not dissolve in water but is soluble in oil. The surfactants are non toxic in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




