
Which one of the following is not an organic acid?
(A) Ethanoic acid
(B) Formic acid
(C) Citric acid
(D) Carbonic acid
Answer
583.2k+ views
Hint: The question is asking to choose an inorganic acid. Recollect the basic fundamentals of organic chemistry. Find out where are the acids given in the options are used in our daily life. Find out which of the acids have carboxyl groups in them and accordingly, mark them as organic or inorganic and then tick the correct option.
Complete step by step solution:
- A compound is said to be organic if it has at least one hydrocarbon chain in it.
- Organic chemistry is basically the chemistry of organs, that is, living beings. All living beings are composed of hydrocarbons as the backbone.
- In organic chemistry, an organic acid will have the functional group as carboxylic acid $\left( -COOH \right)$.
- Let’s now have a look at the acids given in the question.
- Ethanoic acid is also commonly known as acetic acid. In dilute form, it is used as vinegar for cooking. The structure of acetic acid is $C{{H}_{3}}COOH$. Therefore, it is organic.
- Formic acid is methanoic acid. You must have felt an itching sensation when an ant bites you. This is because ant sting contains formic acid which causes irritation. The structure of formic acid is $HCOOH$.
- Citric acid reminds us of citrus fruits. All citrus fruits contain citric acid. Its structure is,
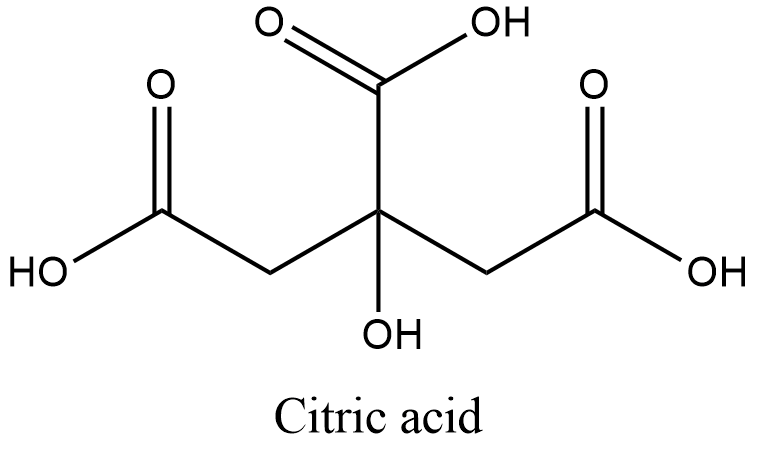
Therefore, citric acid is also an organic acid due to the presence of three carboxylic groups.
- Carbonic acid has the molecular formula, ${{H}_{2}}C{{O}_{3}}$. In carbonic acid, hydrogen atoms are not directly bonded to carbon atoms. Hydrogen atoms are bonded to oxygen atoms to form hydroxyl groups which are attached to carbon atoms. Its structure is given as,
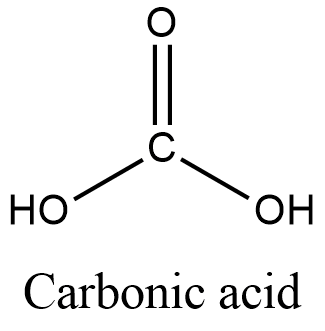
- Although carbonic acid is present in our stomach and part of the digestive process, it doesn’t contain C-H bond and therefore, it is not an organic acid. It is an inorganic acid.
- Therefore, the answer is option (D).
Note: Remember for a compound to be organic in nature, it should have at least one carbon-hydrogen bond. Organic acids will have the presence of a carboxylic acid group. Inorganic acids are the mineral acids or acids which do not contain the presence of carbon-hydrogen bonds.
Complete step by step solution:
- A compound is said to be organic if it has at least one hydrocarbon chain in it.
- Organic chemistry is basically the chemistry of organs, that is, living beings. All living beings are composed of hydrocarbons as the backbone.
- In organic chemistry, an organic acid will have the functional group as carboxylic acid $\left( -COOH \right)$.
- Let’s now have a look at the acids given in the question.
- Ethanoic acid is also commonly known as acetic acid. In dilute form, it is used as vinegar for cooking. The structure of acetic acid is $C{{H}_{3}}COOH$. Therefore, it is organic.
- Formic acid is methanoic acid. You must have felt an itching sensation when an ant bites you. This is because ant sting contains formic acid which causes irritation. The structure of formic acid is $HCOOH$.
- Citric acid reminds us of citrus fruits. All citrus fruits contain citric acid. Its structure is,

Therefore, citric acid is also an organic acid due to the presence of three carboxylic groups.
- Carbonic acid has the molecular formula, ${{H}_{2}}C{{O}_{3}}$. In carbonic acid, hydrogen atoms are not directly bonded to carbon atoms. Hydrogen atoms are bonded to oxygen atoms to form hydroxyl groups which are attached to carbon atoms. Its structure is given as,

- Although carbonic acid is present in our stomach and part of the digestive process, it doesn’t contain C-H bond and therefore, it is not an organic acid. It is an inorganic acid.
- Therefore, the answer is option (D).
Note: Remember for a compound to be organic in nature, it should have at least one carbon-hydrogen bond. Organic acids will have the presence of a carboxylic acid group. Inorganic acids are the mineral acids or acids which do not contain the presence of carbon-hydrogen bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




