
Which one of the following compounds will be most readily dehydrated?
A)
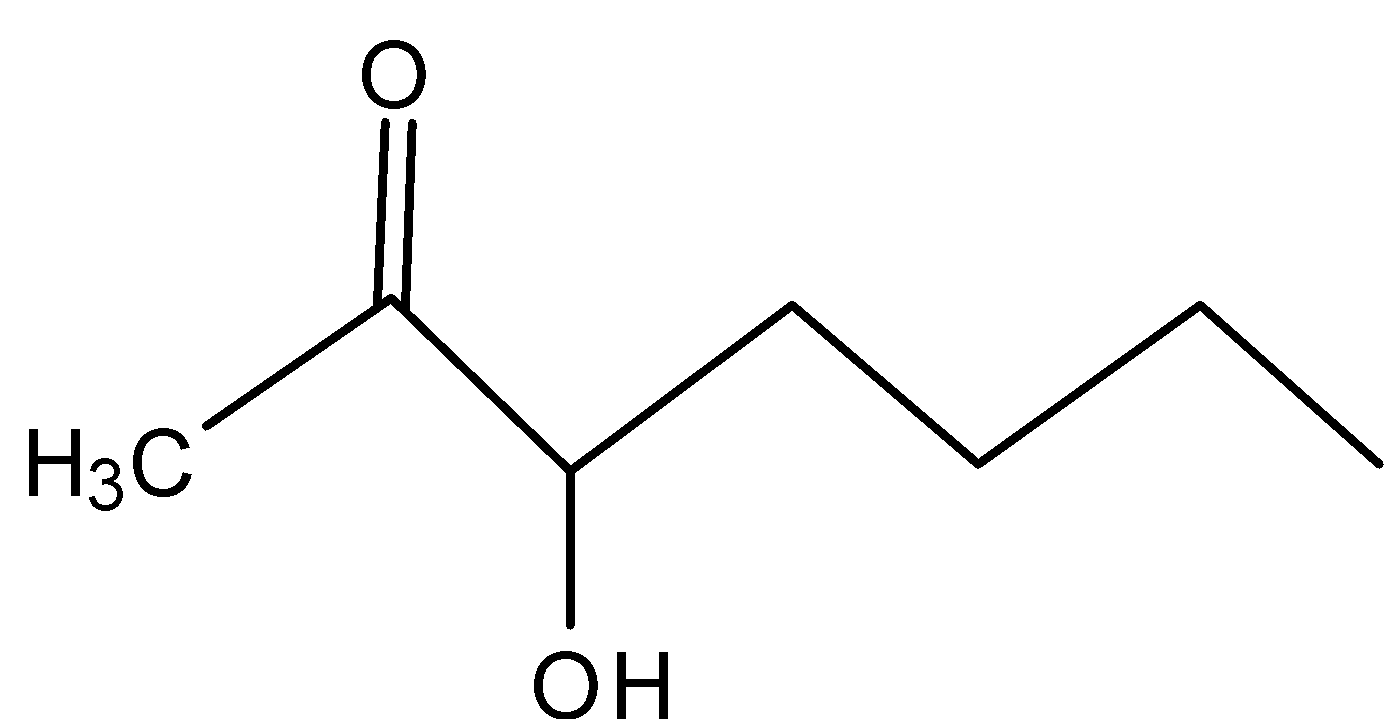
B)
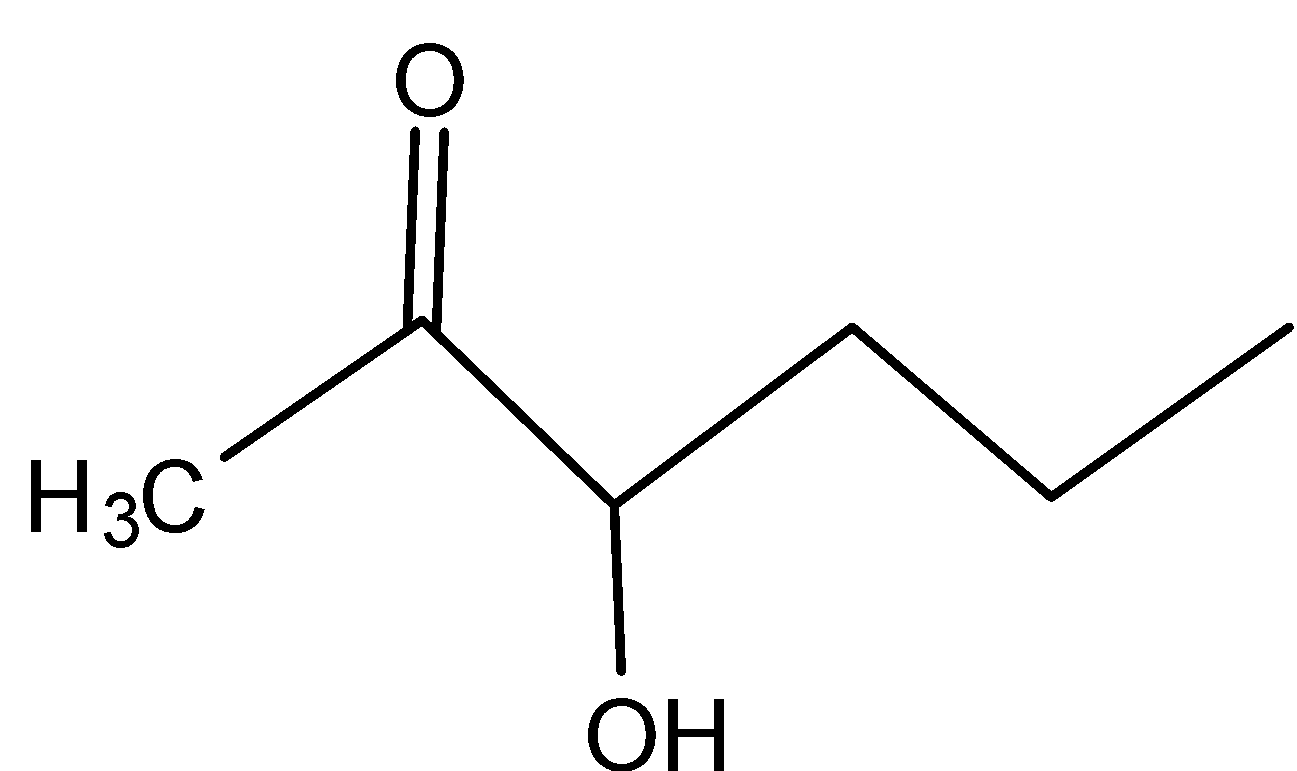
C)
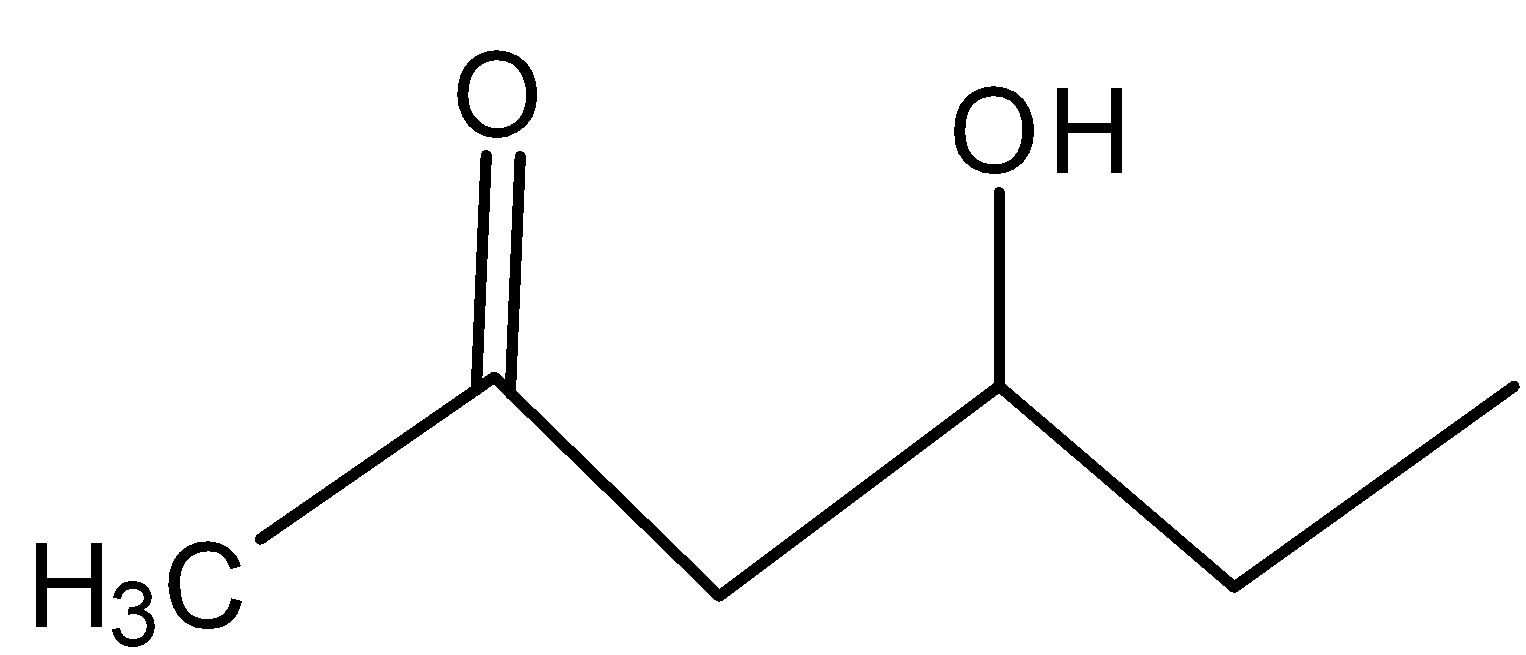
D)
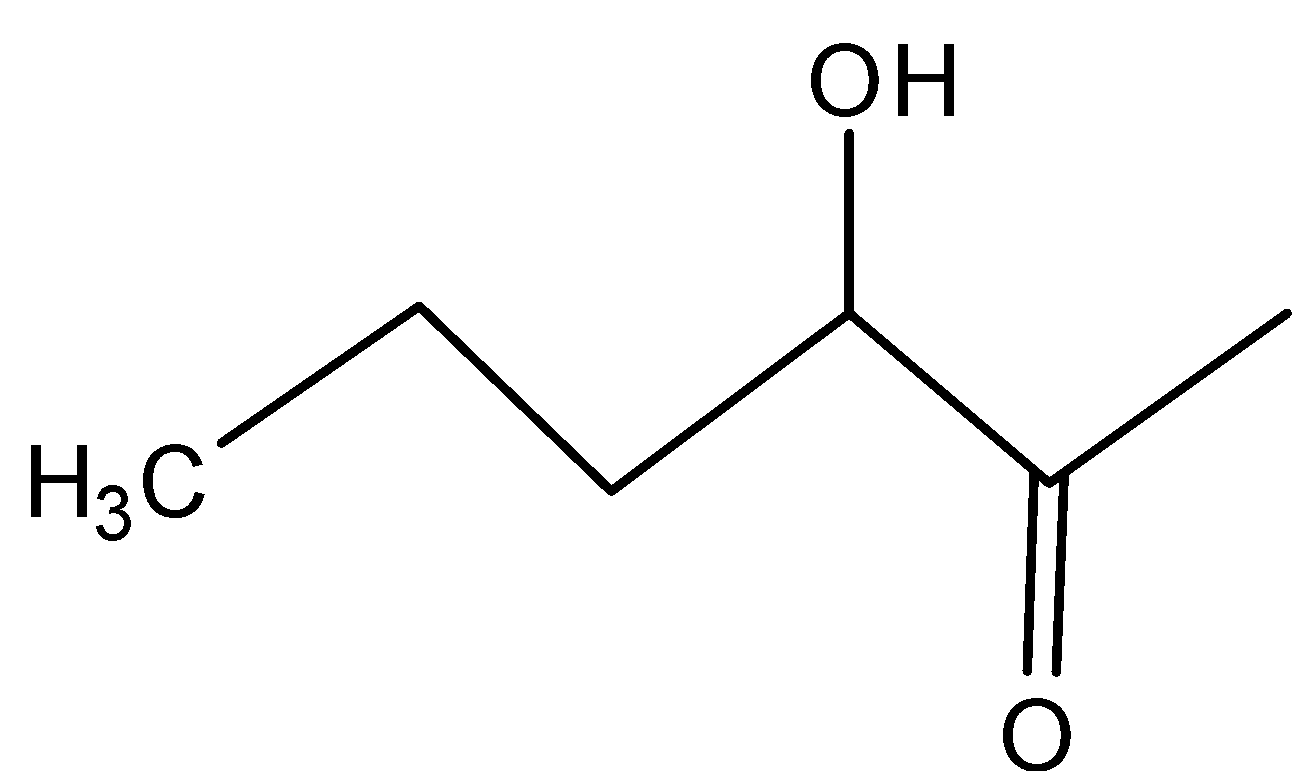




Answer
542.4k+ views
Hint: The answer lies in the fact that the dehydration reaction is nothing but that the hydrogen atom is being removed in a reaction in the form of a water molecule in the presence of a dehydrating agent.
Complete answer:
In the classes of organic chemistry, we have dealt with the topics which tell us about some of the basic reactions such as addition reactions, substitution reactions, elimination reactions, dehydration reactions, hydrogenation reactions and so on.
Let us see about the dehydration of each of the compounds given in detail so that we can understand which compound can undergo easy dehydration.
Dehydration reactions basically involve the formation of the carbocation and the most stable the carbocation is more easily the compound is dehydrated.
In A)
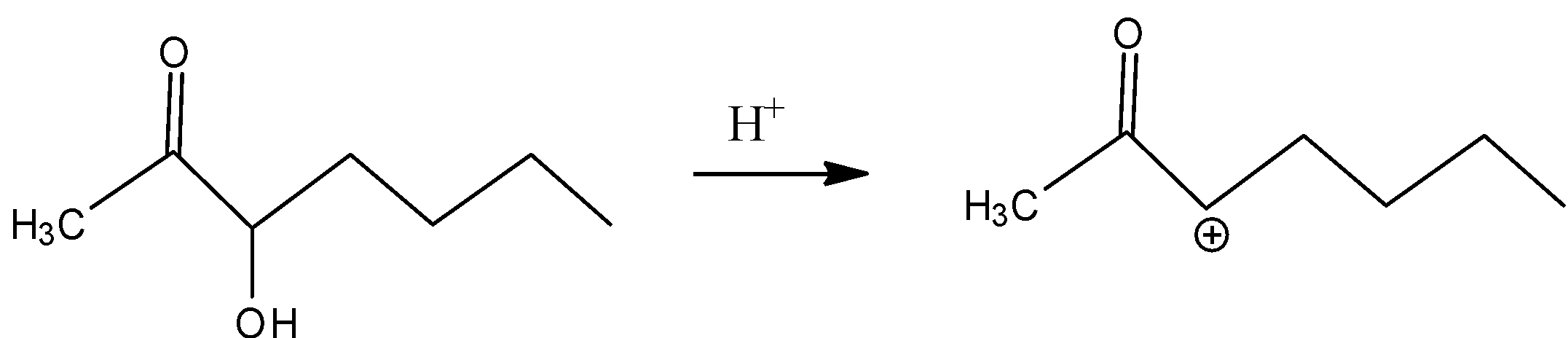
The carbocation formed is the secondary carbocation which is more stable than primary carbocation, the –I effect makes it less stable because of the presence of carbonyl groups adjacent to the carbocation.
In B)
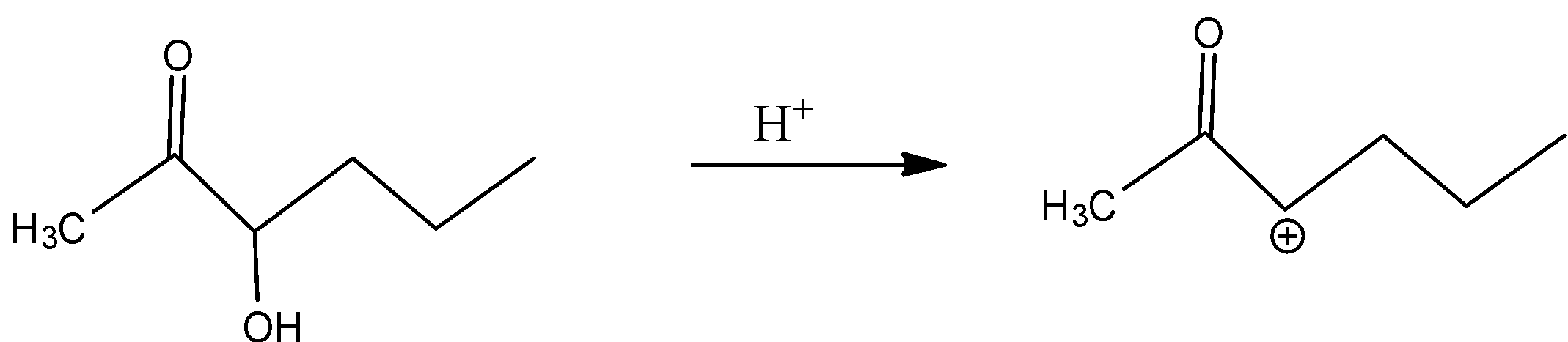
Even here, even if the secondary carbocation is present, the inductive effect is present because of the adjacent carbonyl group.
In C)
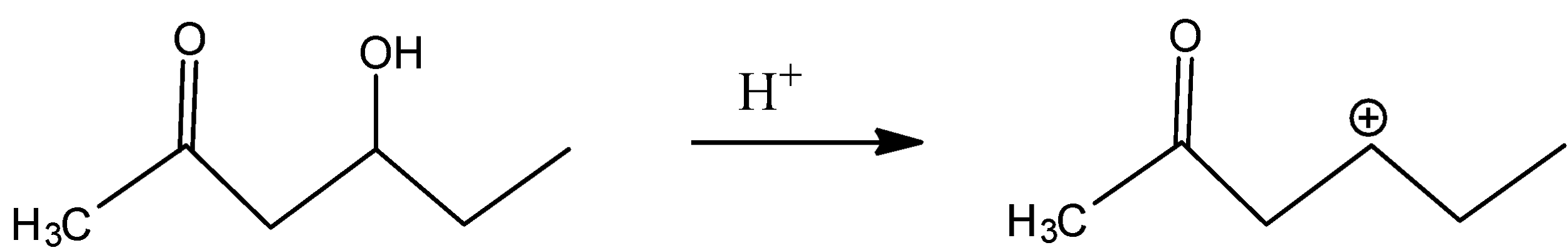
The carbocation present is far from the inductive effect possessed by the carbonyl group and thus is dehydrated readily.
In D)
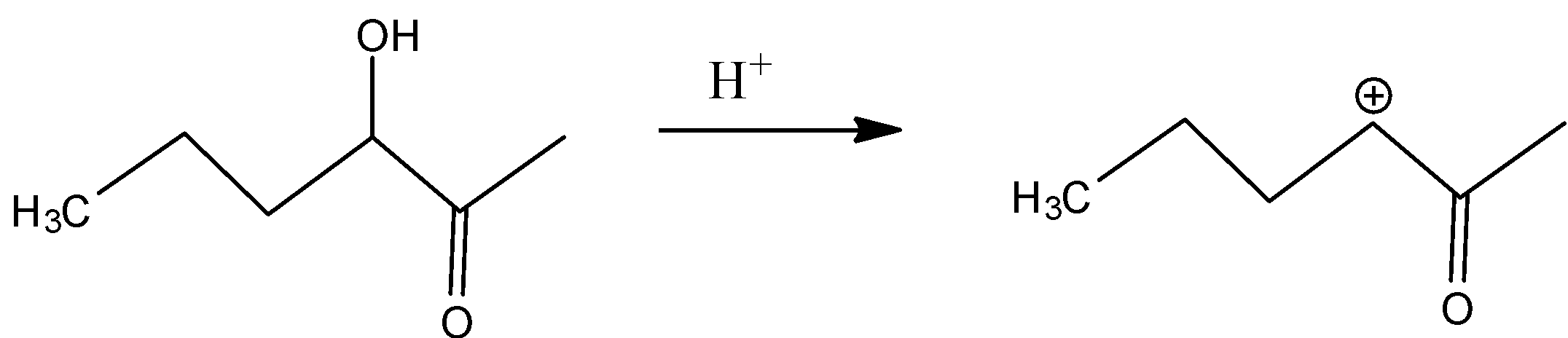
In this, the same inductive effect plays a role which is more compared to the compound in option and hence is not readily dehydrated.
Thus, the correct answer is option C)
Note:
Note that the stability of the carbocation is always directly proportional to the + inductive effect and + mesomeric effect whereas it is inversely proportional to the = inductive and – mesomeric effect.
Complete answer:
In the classes of organic chemistry, we have dealt with the topics which tell us about some of the basic reactions such as addition reactions, substitution reactions, elimination reactions, dehydration reactions, hydrogenation reactions and so on.
Let us see about the dehydration of each of the compounds given in detail so that we can understand which compound can undergo easy dehydration.
Dehydration reactions basically involve the formation of the carbocation and the most stable the carbocation is more easily the compound is dehydrated.
In A)

The carbocation formed is the secondary carbocation which is more stable than primary carbocation, the –I effect makes it less stable because of the presence of carbonyl groups adjacent to the carbocation.
In B)

Even here, even if the secondary carbocation is present, the inductive effect is present because of the adjacent carbonyl group.
In C)

The carbocation present is far from the inductive effect possessed by the carbonyl group and thus is dehydrated readily.
In D)

In this, the same inductive effect plays a role which is more compared to the compound in option and hence is not readily dehydrated.
Thus, the correct answer is option C)
Note:
Note that the stability of the carbocation is always directly proportional to the + inductive effect and + mesomeric effect whereas it is inversely proportional to the = inductive and – mesomeric effect.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




