
Which one of the following complexes will have six isomers?
A.\[\;\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_2}C{l_2}} \right]Cl\]
B.\[\;\left[ {Cr{{\left( {{H_2}O} \right)}_4}C{l_2}} \right]Cl\]
C.\[{\left[ {Co{{\left( {ox} \right)}_3}} \right]^{3 - }}\]
D.\[\left[ {Co{{\left( {en} \right)}_2}B{r_2}} \right]Cl\]
Answer
561.3k+ views
Hint:The geometric isomers are possible for both square planar and octahedral complexes, but not tetrahedral and the optical isomers are possible for both tetrahedral and octahedral complexes, but not square planar.
Complete answer:
Two or more different compounds which have the same formula are known as isomers. The metal complexes that differ only in which ligands are adjacent to one another i.e. cis or directly across from one another i.e. trans in the coordination sphere of the metal are known as geometrical isomers. They are the most important for square planar and octahedral complexes.
Optical isomers are related as non-superimposable mirror images and differ in the direction with which they rotate the plane-polarised light. These isomers are referred to as enantiomers or enantiomorphs of each other and their non-superimposable structures are said to be asymmetric.
In the year 1911, the first resolution of optical isomers was reported by Werner and King for the complexes cis-\[{\left[ {CoX\left( {N{H_3}} \right){{\left( {en} \right)}_2}} \right]^{2 + }}\], where \[X = C{l^ - }\] or \[B{r^ - }\].
When we see the isomers of \[\;\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_2}C{l_2}} \right]Cl\], (i) and (ii) have cis-optical isomer also so the total isomers are 4 geometrical and 2 optical isomers which makes it to having six isomers.
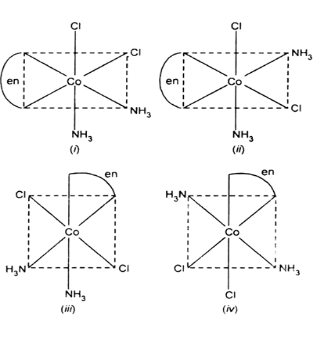
Therefore, the correct answer is option (A).
Note:
The existence of coordination compounds with the same formula but with the different arrangements of the ligands was very crucial in the development of coordination chemistry. Two or more compounds which have the same formula but having different arrangements of the atoms are known as isomers. As isomers usually have different physical and chemical properties, so it becomes very important for us to know which isomer we are dealing with if more than one isomer is possible.
Complete answer:
Two or more different compounds which have the same formula are known as isomers. The metal complexes that differ only in which ligands are adjacent to one another i.e. cis or directly across from one another i.e. trans in the coordination sphere of the metal are known as geometrical isomers. They are the most important for square planar and octahedral complexes.
Optical isomers are related as non-superimposable mirror images and differ in the direction with which they rotate the plane-polarised light. These isomers are referred to as enantiomers or enantiomorphs of each other and their non-superimposable structures are said to be asymmetric.
In the year 1911, the first resolution of optical isomers was reported by Werner and King for the complexes cis-\[{\left[ {CoX\left( {N{H_3}} \right){{\left( {en} \right)}_2}} \right]^{2 + }}\], where \[X = C{l^ - }\] or \[B{r^ - }\].
When we see the isomers of \[\;\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_2}C{l_2}} \right]Cl\], (i) and (ii) have cis-optical isomer also so the total isomers are 4 geometrical and 2 optical isomers which makes it to having six isomers.

Therefore, the correct answer is option (A).
Note:
The existence of coordination compounds with the same formula but with the different arrangements of the ligands was very crucial in the development of coordination chemistry. Two or more compounds which have the same formula but having different arrangements of the atoms are known as isomers. As isomers usually have different physical and chemical properties, so it becomes very important for us to know which isomer we are dealing with if more than one isomer is possible.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




