
Which one is not an Electrophile
(A) $ B{F_3} $
(B) $ N{H_3} $
(C) $ S{O_3} $
(D) $ AlC{l_3} $
Answer
506.1k+ views
Hint :An electrophile is an atom, or molecule that in chemical reaction seeks an atom or molecule containing an electron pair available for bonding. Electrophile are electron deficient species and can accept an electron pair from electron rich species.
Complete Step By Step Answer:
Lewis acids are by definition electrophiles. According to the Bronsted-Lewis concept an acid is a substance that requires electrons and can accept lone pairs and behaves as an electrophile.
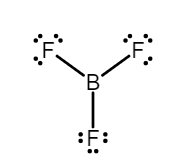
$ (1)B{F_3} $ - Boron trifluoride is an electrophile because boron has an empty $ p $ orbitals, because it is an electron deficient species and has $ 3 $ electrons coupled with $ 3 $ electrons coming from three fluorine atoms, in total $ 6 $ electrons involved in this compound. Therefore, $ 2 $ electrons can be accommodated in the molecule. Hence, it is an electrophile.
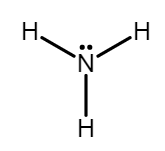
$ (2)N{H_3} $ - Ammonia has a lone pair of electrons and a $ {\delta ^ - } $ charge on the nitrogen atom, which allows it to be a nucleophile since it can provide electrons to form a new bond. A nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. Ammonia doesn’t carry a negative charge. But it has a lone pair of electrons, and nitrogen is more electronegative than hydrogen, so nitrogen molecules with a $ {\delta ^ - } $ charge somewhere.
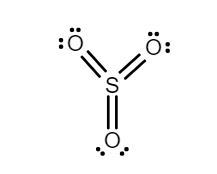
$ (3)S{O_3} $ - Sulphur trioxide is an electrophile because Sulphur is bonded to three oxygen, twice each. The Sulphur in the middle has a formal charge of zero, but the oxygens it is bonded to are extremely electronegative; this makes Sulphur electron deficient, and so the Sulphur atom in the middle has a partial charge plus charge on it. Therefore, it will be willing to accept electrons. It is a good Lewis acid.
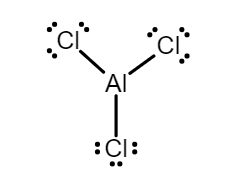
$ (4)AlC{l_3} $ - Aluminum chloride is an electrophile, as it looks like an ionic compound but it’s a covalent compound. According to octet rule aluminum should have eight electrons in the outermost shell but there are only six electrons after bonding with chlorine as each chlorine shares one electron with aluminum. Owing to deficiency of electrons it acts as an electrophile.
Option (B) is correct.
Note :
Boron trifluoride is a strong Lewis acid that acts as a catalyst for organic synthesis reactions, it reacts rapidly with chemicals containing oxygen, nitrogen, Sulphur and other electron pair donors.
Ammonia is used as refrigerant gas, for production of water supplies and in manufacture of plastics, explosive, textiles, pesticide, dyes and other chemicals.
Sulphur trioxide is used in the production of sulfuric acid and other chemicals, and explosives.
Aluminum chloride is used widely in manufacturing rubber, lubricants, wood preservatives and paints. It also acts as a catalyst.
Complete Step By Step Answer:
Lewis acids are by definition electrophiles. According to the Bronsted-Lewis concept an acid is a substance that requires electrons and can accept lone pairs and behaves as an electrophile.
$ (1)B{F_3} $ - Boron trifluoride is an electrophile because boron has an empty $ p $ orbitals, because it is an electron deficient species and has $ 3 $ electrons coupled with $ 3 $ electrons coming from three fluorine atoms, in total $ 6 $ electrons involved in this compound. Therefore, $ 2 $ electrons can be accommodated in the molecule. Hence, it is an electrophile.

$ (2)N{H_3} $ - Ammonia has a lone pair of electrons and a $ {\delta ^ - } $ charge on the nitrogen atom, which allows it to be a nucleophile since it can provide electrons to form a new bond. A nucleophile is a reactant that provides a pair of electrons to form a new covalent bond. Ammonia doesn’t carry a negative charge. But it has a lone pair of electrons, and nitrogen is more electronegative than hydrogen, so nitrogen molecules with a $ {\delta ^ - } $ charge somewhere.

$ (3)S{O_3} $ - Sulphur trioxide is an electrophile because Sulphur is bonded to three oxygen, twice each. The Sulphur in the middle has a formal charge of zero, but the oxygens it is bonded to are extremely electronegative; this makes Sulphur electron deficient, and so the Sulphur atom in the middle has a partial charge plus charge on it. Therefore, it will be willing to accept electrons. It is a good Lewis acid.

$ (4)AlC{l_3} $ - Aluminum chloride is an electrophile, as it looks like an ionic compound but it’s a covalent compound. According to octet rule aluminum should have eight electrons in the outermost shell but there are only six electrons after bonding with chlorine as each chlorine shares one electron with aluminum. Owing to deficiency of electrons it acts as an electrophile.

Option (B) is correct.
Note :
Boron trifluoride is a strong Lewis acid that acts as a catalyst for organic synthesis reactions, it reacts rapidly with chemicals containing oxygen, nitrogen, Sulphur and other electron pair donors.
Ammonia is used as refrigerant gas, for production of water supplies and in manufacture of plastics, explosive, textiles, pesticide, dyes and other chemicals.
Sulphur trioxide is used in the production of sulfuric acid and other chemicals, and explosives.
Aluminum chloride is used widely in manufacturing rubber, lubricants, wood preservatives and paints. It also acts as a catalyst.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




