
Which of the statements is correct for the given acids?
A) Phosphinic acid is a diprotic acid while Phosphonic acid is a monoprotic acid
B) Phosphinic acid is a monoprotic acid while Phosphonic acid is a diprotic acid
C) Both are triprotic acids
D) Both are diprotic acids
Answer
562.5k+ views
Hint: We know that a diprotic acid is an acid that can donate two hydrogen ions $({H^ + })$ or protons per molecule in an aqueous solution, monoprotic acid is an acid that can donate only one proton, while polyprotic acid can donate more than one proton and triprotic acid is the one that can donate three hydrogen ions per molecule during dissociation.
Complete answer:
We will first draw the structures of both the acids and from there we will identify the number of hydrogen ions.
Structure of Phosphinic and Phosphonic acid:
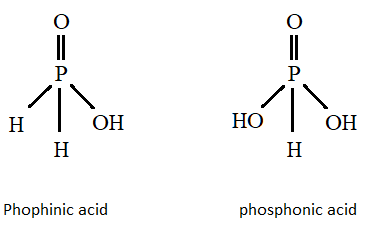
Here we can see that phosphinic acid can donate two hydrogen ions so it is known as diprotic acid whereas phosphonic acid can only donate one hydrogen atom so it will come under monoprotic acid.
Explanation of diprotic and monoprotic acid:
Diprotic acid is also called dibasic acid. A diprotic acid is an acid which is able to donate more than one proton. Whereas a monoprotic acid only donates one proton or hydrogen in water. Some examples of monoprotic acids are $HCl,HF,HBr,HN{O_3}$ .Some examples of diprotic acids are ${H_2}S{O_4},{H_2}S,{H_2}Cr{O_4}$
Therefore, the correct answer is option A
Note: Phosphinic acid is a phosphorus oxyacid and is also called hypo phosphorous acid $({H_3}P{O_3})$. It is a powerful reducing agent and is used for the reduction of arene diazonium salts. It is also used for electro less nickel plating in industries. Phosphonic acids are diprotic acids and are mostly used for the synthesis of basic lead phosphite. They are effective as chelating agents.
Complete answer:
We will first draw the structures of both the acids and from there we will identify the number of hydrogen ions.
Structure of Phosphinic and Phosphonic acid:

Here we can see that phosphinic acid can donate two hydrogen ions so it is known as diprotic acid whereas phosphonic acid can only donate one hydrogen atom so it will come under monoprotic acid.
Explanation of diprotic and monoprotic acid:
Diprotic acid is also called dibasic acid. A diprotic acid is an acid which is able to donate more than one proton. Whereas a monoprotic acid only donates one proton or hydrogen in water. Some examples of monoprotic acids are $HCl,HF,HBr,HN{O_3}$ .Some examples of diprotic acids are ${H_2}S{O_4},{H_2}S,{H_2}Cr{O_4}$
Therefore, the correct answer is option A
Note: Phosphinic acid is a phosphorus oxyacid and is also called hypo phosphorous acid $({H_3}P{O_3})$. It is a powerful reducing agent and is used for the reduction of arene diazonium salts. It is also used for electro less nickel plating in industries. Phosphonic acids are diprotic acids and are mostly used for the synthesis of basic lead phosphite. They are effective as chelating agents.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




