
Which of the following will not give an alkane on treatment with \[2-chloro-2-methylbutane\] ?
A. $NaB{{H}_{4}}$
B. $LiAl{{H}_{4}}$
C. ${{({{C}_{6}}{{H}_{5}})}_{3}}SnH$
D. $(i)Mg/ether,(ii){{H}_{3}}{{O}^{+}}$
Answer
535.8k+ views
Hint: We know that the phenomenon of existence with the same molecule formula of two or more compounds but exhibit different physical and chemical properties is known as isomers and the compound which shows isomerism phenomenon is an isomer, based on the organic compounds and its functional groups, this isomerism is mainly classified into structural and stereoisomerism. Chlorination of alkane undergoes in the presence of UV-light.
Complete step by step answer:
Monochlorination of alkanes means the substitution of one chlorine atom by replacing one hydrogen atom. This reaction is a radical substitution reaction in alkanes. The number of monochloro isomers depends on the types of hydrogen atoms present. The structure of
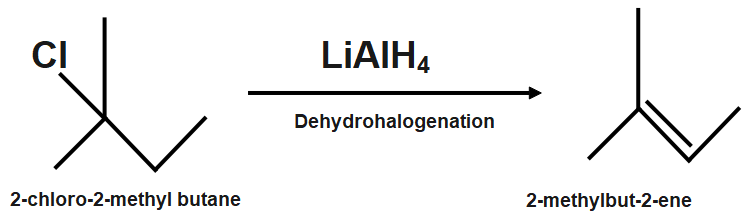
Different types of hydrogen atoms will present like, there are four types of hydrogen atoms as one type of two primary hydrogen atoms, one type of secondary hydrogen atom, and one type of tertiary hydrogen atom. Because of the four different hydrogen atoms there is a possibility of replacement of hydrogen atom with chlorine atom during monochlorination six structural isomers will form as monochloro products.
So, the correct answer is Option B.
Note: There are three types of structural isomers observed in alkanes. They are functional group isomers, chain isomers, functional group isomers, and positional isomers. When monochlorination occurs in alkanes, the possibility of constitutional isomers depends on the structural formula and the orientation of hydrogen molecules attached to carbon atoms.
Complete step by step answer:
Monochlorination of alkanes means the substitution of one chlorine atom by replacing one hydrogen atom. This reaction is a radical substitution reaction in alkanes. The number of monochloro isomers depends on the types of hydrogen atoms present. The structure of

Different types of hydrogen atoms will present like, there are four types of hydrogen atoms as one type of two primary hydrogen atoms, one type of secondary hydrogen atom, and one type of tertiary hydrogen atom. Because of the four different hydrogen atoms there is a possibility of replacement of hydrogen atom with chlorine atom during monochlorination six structural isomers will form as monochloro products.
So, the correct answer is Option B.
Note: There are three types of structural isomers observed in alkanes. They are functional group isomers, chain isomers, functional group isomers, and positional isomers. When monochlorination occurs in alkanes, the possibility of constitutional isomers depends on the structural formula and the orientation of hydrogen molecules attached to carbon atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




