
Which of the following will have a dipole moment?
A)
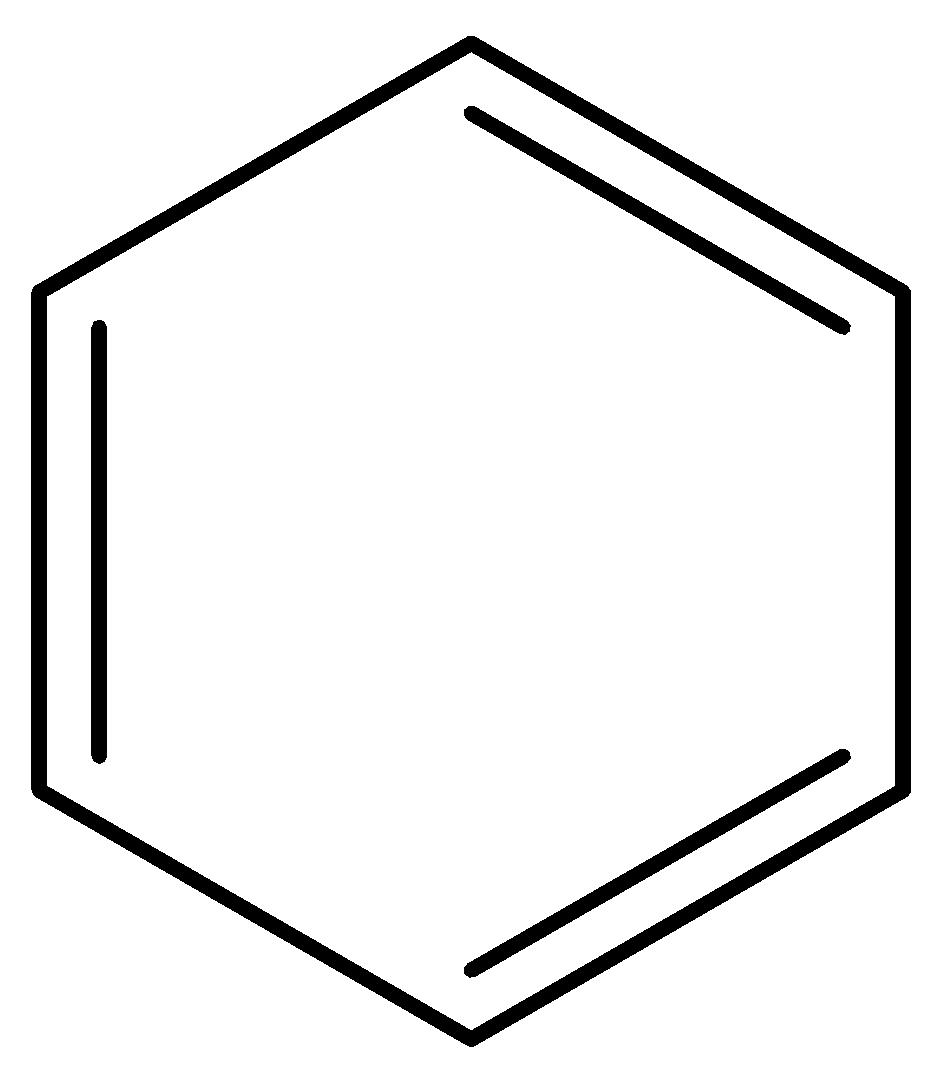
B)
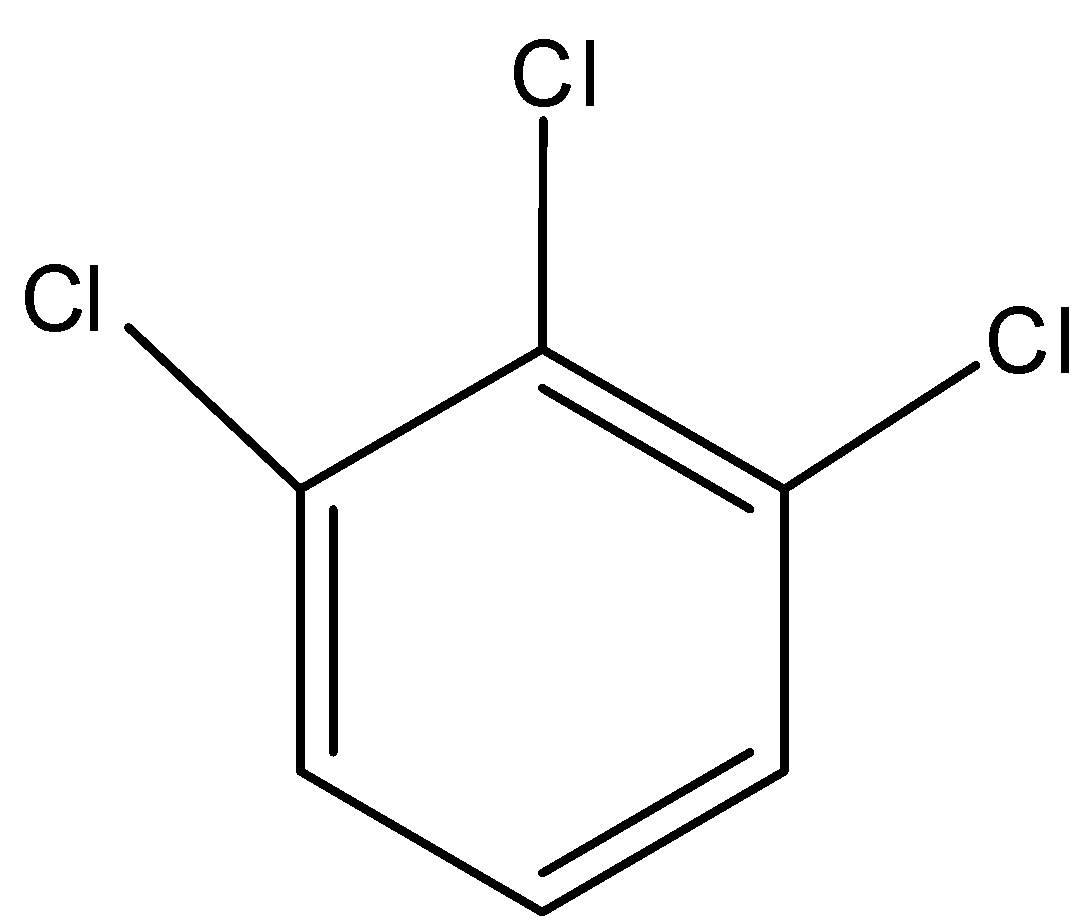
C)
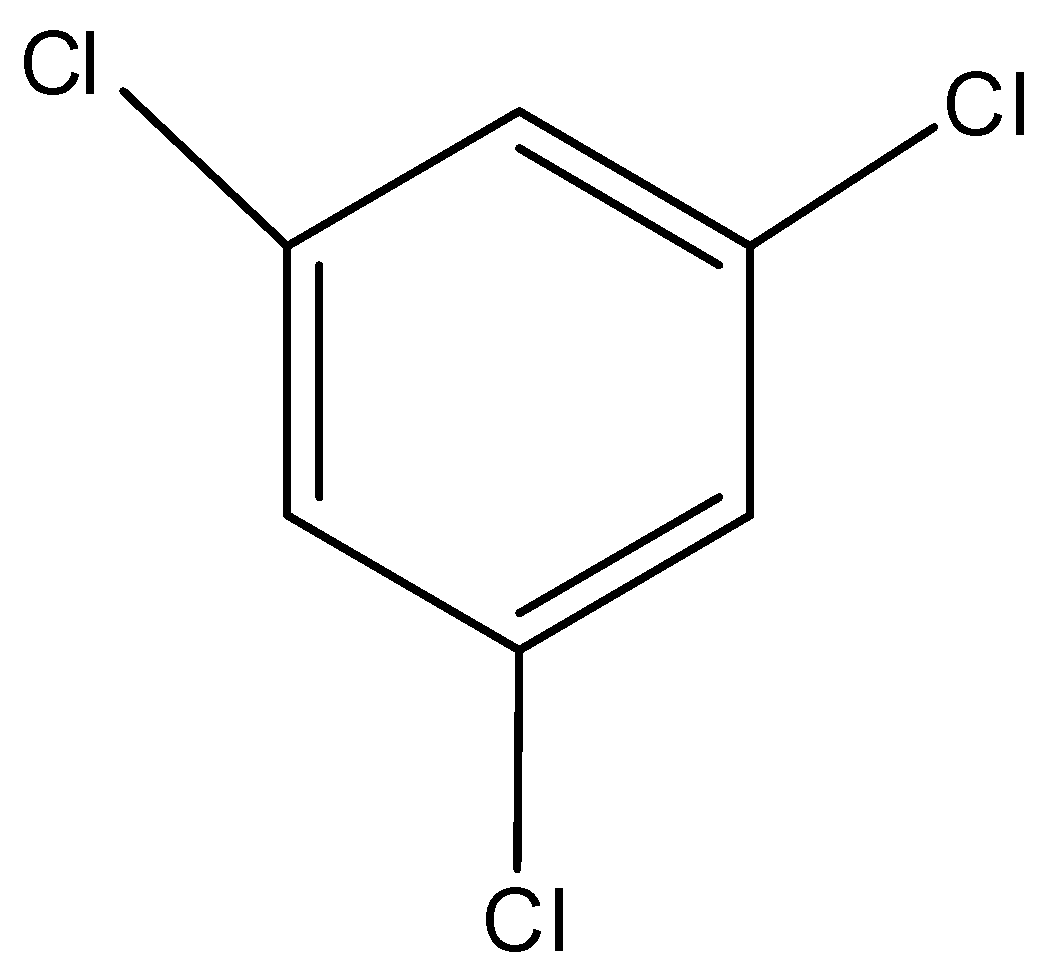
D)
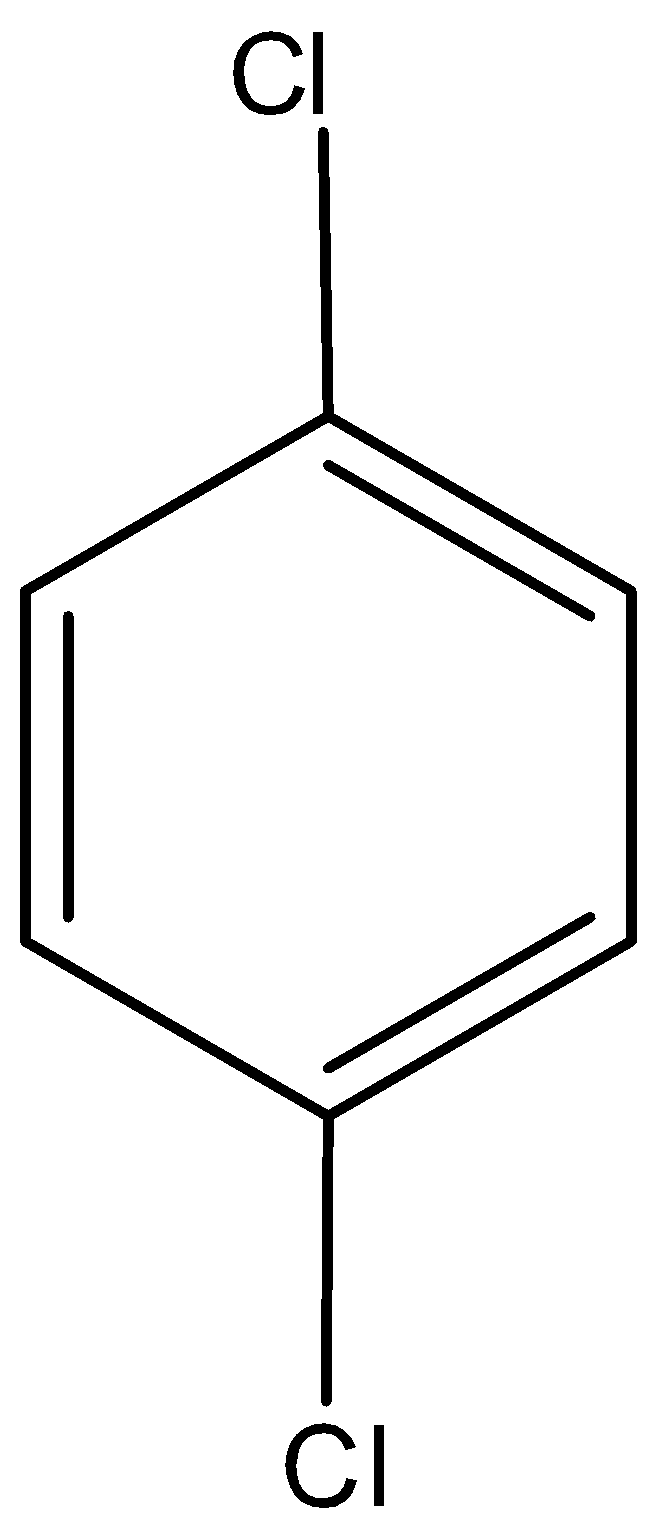




Answer
559.5k+ views
Hint: We realize that a moment emerges in any framework during which there's a partition of charge is known as a dipole moment. They can, therefore, arise in ionic bonds also as in covalent bonds. Dipole moments arise due to the difference in electronegativity between two chemically bonded atoms.
Complete step by step answer:
We need to remember that the dipole moment of Benzene is generally zeros. Particles having a dipole second are either uneven or have varying electronegativities. While carbon and hydrogen have various electronegativities, the benzene atom is a balanced planar particle. It has 6 C-H bonds or 6 dipole minutes, which offset each other and consequently as all individual dipoles counteract, the net dipole second is zero. Hence option A is incorrect.
The dipole moment of para dichlorobenzene is zero due to symmetrical structure. Hence, option D is incorrect.
The dipole second is a component of position and bond snapshot of ionic components. Subsequently, for 1, 2, 3, trichloro benzene the dipole second is highly contrasted with others and for the 1, 3, 5 trichlorobenzene.
So, the correct answer is Option C.
Note: We know that polarity results from the uneven partial charge distribution between various atoms in a compound. Atoms similar to nitrogen, oxygen, and incandescent lamps that are more electronegative will in general have halfway negative charges. Molecules, similar to carbon and hydrogen, will in general be more unbiased or have halfway sure charges.
The polar nature of the molecules can be measured by the dipole moment. A molecule with zero dipole moment then it is a nonpolar molecule. If a molecule has a net dipole moment then it is a polar molecule.
Complete step by step answer:
We need to remember that the dipole moment of Benzene is generally zeros. Particles having a dipole second are either uneven or have varying electronegativities. While carbon and hydrogen have various electronegativities, the benzene atom is a balanced planar particle. It has 6 C-H bonds or 6 dipole minutes, which offset each other and consequently as all individual dipoles counteract, the net dipole second is zero. Hence option A is incorrect.
The dipole moment of para dichlorobenzene is zero due to symmetrical structure. Hence, option D is incorrect.
The dipole second is a component of position and bond snapshot of ionic components. Subsequently, for 1, 2, 3, trichloro benzene the dipole second is highly contrasted with others and for the 1, 3, 5 trichlorobenzene.
So, the correct answer is Option C.
Note: We know that polarity results from the uneven partial charge distribution between various atoms in a compound. Atoms similar to nitrogen, oxygen, and incandescent lamps that are more electronegative will in general have halfway negative charges. Molecules, similar to carbon and hydrogen, will in general be more unbiased or have halfway sure charges.
The polar nature of the molecules can be measured by the dipole moment. A molecule with zero dipole moment then it is a nonpolar molecule. If a molecule has a net dipole moment then it is a polar molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




