
Which of the following will give propyne on hydrolysis?
A.$A{l_4}{C_3}$
B.$M{g_2}{C_3}$
C.${B_4}C$
D.$L{a_4}{C_3}$
Answer
548.7k+ views
Hint: Propyne is an alkyne. For organic synthesis, Propyne is a convenient three-carbon construction block with four hydrogen atoms having a triple bond between two carbon atoms. In order to find the compound which will give propyne on hydrolysis we must write the hydrolysis reaction of each of the compounds given. And from the product obtained you will come to know which compound gives propyne.
Complete step by step answer:
Let us first understand hydrolysis;
Hydrolysis is a chemical reaction where the water molecules are added to the compound undergoing the reaction. During hydrolysis the water molecule undergoes decomposition to give ${H^ + }$ and $O{H^ - }$ions respectively.
$\,HOH\, \rightleftarrows {H^ + } + O{H^ - }\,$
All the given options are examples of metal carbides.
Now, we know that carbides are compounds that have carbon and other elements which have less electronegative character than carbon atoms.
Metal carbides are the compounds which have a transition metal ion and carbon atom as the constituents. These structures are more of a complex type. Propyne is basically, $\,{C_3}{H_4}\,$
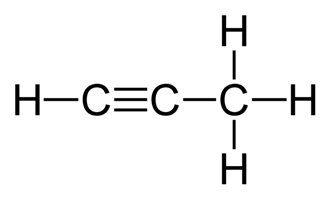
Now, we must perform hydrolysis on the given options to see which compound will give propyne:
In option A, $A{l_4}{C_3}$ is a metal carbide and on hydrolysis yields methane and aluminium hydroxide.
$A{l_3}C{ _4} + 12HOH \to 3C{H_4} + 4Al{(OH)_3}$
So, option A is the incorrect answer.
In option B, $M{g_2}{C_3}$ is an ionic carbide and on hydrolysis with water yields magnesium hydroxide and propyne which is a three carbon product.
$M{g_2}{C_3} + 4{H_2}O \to 2Mg{(OH)_2} + {C_3}{H_4}$
So, option B is correct.
In option C, ${B_4}C$ on hydrolysis gives boron oxide while carbon monoxide and hydrogen gases are evolved.
$\,{B_4}C + 7{H_2}O \to 2{B_2}{O_3} + CO + 7{H_2}\,$
So, option C is the incorrect answer.
In option D, $L{a_4}{C_3}$ on hydrolysis gives lanthanum hydroxide and methane.
\[L{a_4}{C_3} + HOH \to La{(OH)_3} + C{H_4}\]
So, option D is also the incorrect answer.
Thus, from the above chemical reactions it is clear that $M{g_2}{C_3}$ is the correct option. As it gives propyne as a product.
Hence, option B is the correct answer to this question.
We should also remember that propyne is also called as allylene.
Note:
$\,M{g_2}{C_3}\,$ hydrolysis yields a combination of propyne and propadiene, the ratio of which is similar to ambient and higher temperature equilibrium levels, while at low temperatures it changes towards propadiene. The hydrolysis reaction's rigorousness complies well with the structural consequences of polycentre, electron-deficient bonds.
Complete step by step answer:
Let us first understand hydrolysis;
Hydrolysis is a chemical reaction where the water molecules are added to the compound undergoing the reaction. During hydrolysis the water molecule undergoes decomposition to give ${H^ + }$ and $O{H^ - }$ions respectively.
$\,HOH\, \rightleftarrows {H^ + } + O{H^ - }\,$
All the given options are examples of metal carbides.
Now, we know that carbides are compounds that have carbon and other elements which have less electronegative character than carbon atoms.
Metal carbides are the compounds which have a transition metal ion and carbon atom as the constituents. These structures are more of a complex type. Propyne is basically, $\,{C_3}{H_4}\,$

Now, we must perform hydrolysis on the given options to see which compound will give propyne:
In option A, $A{l_4}{C_3}$ is a metal carbide and on hydrolysis yields methane and aluminium hydroxide.
$A{l_3}C{ _4} + 12HOH \to 3C{H_4} + 4Al{(OH)_3}$
So, option A is the incorrect answer.
In option B, $M{g_2}{C_3}$ is an ionic carbide and on hydrolysis with water yields magnesium hydroxide and propyne which is a three carbon product.
$M{g_2}{C_3} + 4{H_2}O \to 2Mg{(OH)_2} + {C_3}{H_4}$
So, option B is correct.
In option C, ${B_4}C$ on hydrolysis gives boron oxide while carbon monoxide and hydrogen gases are evolved.
$\,{B_4}C + 7{H_2}O \to 2{B_2}{O_3} + CO + 7{H_2}\,$
So, option C is the incorrect answer.
In option D, $L{a_4}{C_3}$ on hydrolysis gives lanthanum hydroxide and methane.
\[L{a_4}{C_3} + HOH \to La{(OH)_3} + C{H_4}\]
So, option D is also the incorrect answer.
Thus, from the above chemical reactions it is clear that $M{g_2}{C_3}$ is the correct option. As it gives propyne as a product.
Hence, option B is the correct answer to this question.
We should also remember that propyne is also called as allylene.
Note:
$\,M{g_2}{C_3}\,$ hydrolysis yields a combination of propyne and propadiene, the ratio of which is similar to ambient and higher temperature equilibrium levels, while at low temperatures it changes towards propadiene. The hydrolysis reaction's rigorousness complies well with the structural consequences of polycentre, electron-deficient bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




