
Which of the following will give phenol with \[{\text{CaO}}\] and \[{\text{NaOH}}\]?
A. Salicylic acid
B. Picric acid
C. Benzoic acid
D. Amino acid
Answer
594k+ views
Hint: First we remember that phenol is an aromatic organic compound with the molecular formula\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{OH}}\].
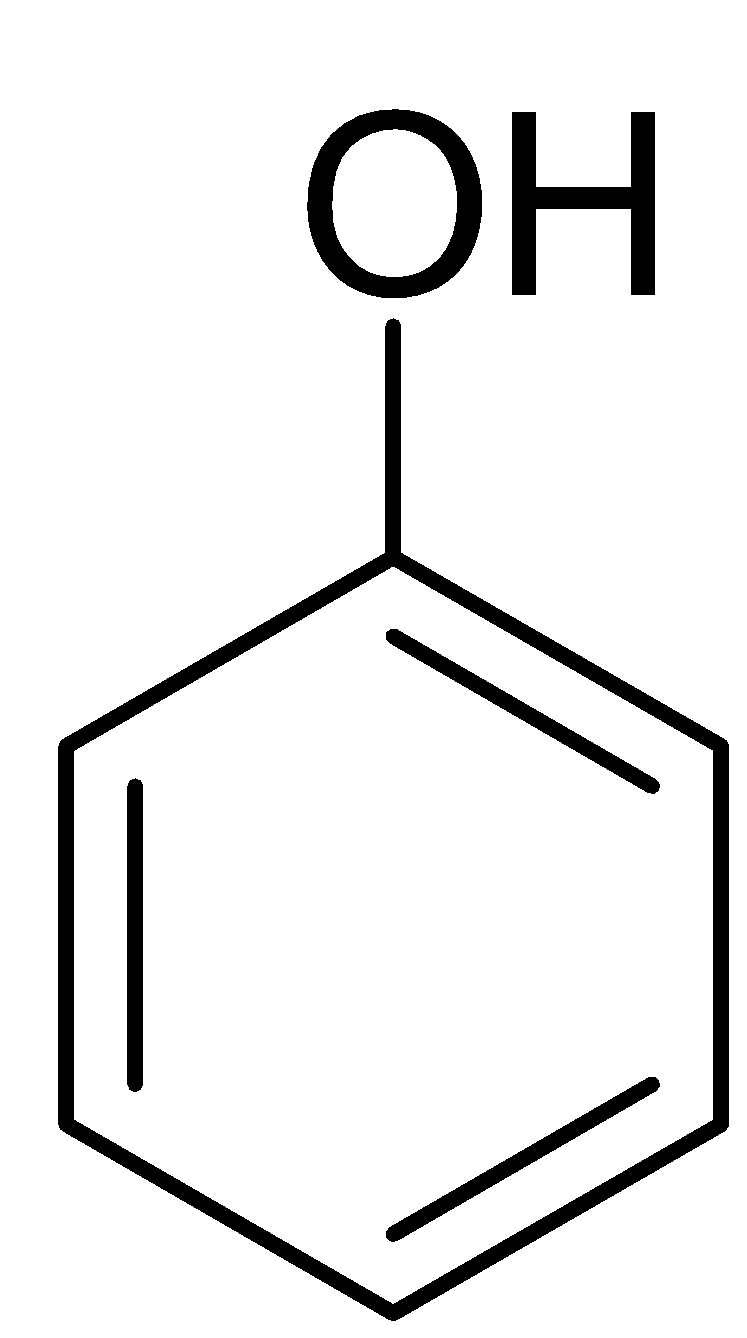
Only salicylic acid (2-Hydroxybenzoic acid) on decarboxylation gives phenol.
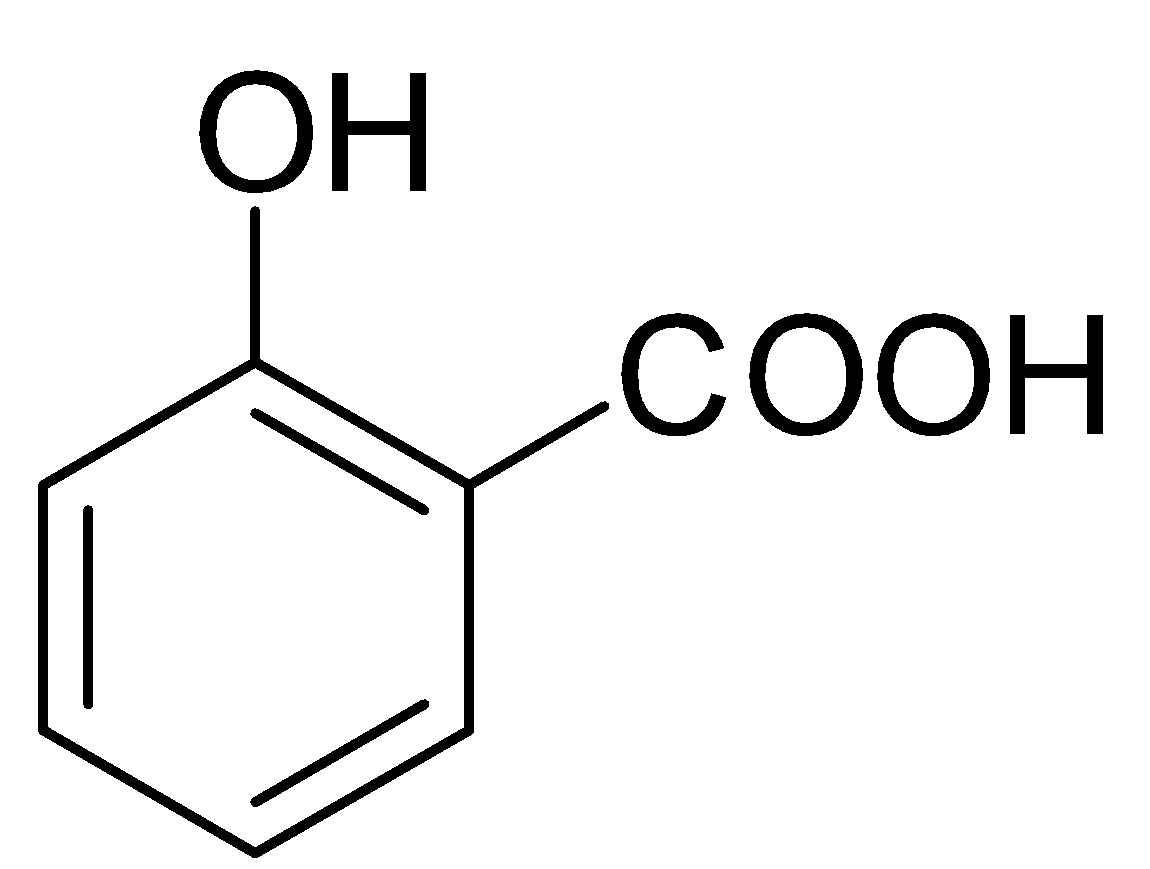
Complete answer:
We must know that salicylic acid will give phenol with \[{\text{CaO}}\] and \[{\text{NaOH}}\]. The mixture of \[{\text{CaO}}\] and \[{\text{NaOH}}\] is called soda lime. When we heat carboxylic acids with soda lime, they undergo decarboxylation. The by-products in this reaction apart from phenol are sodium carbonate and water.
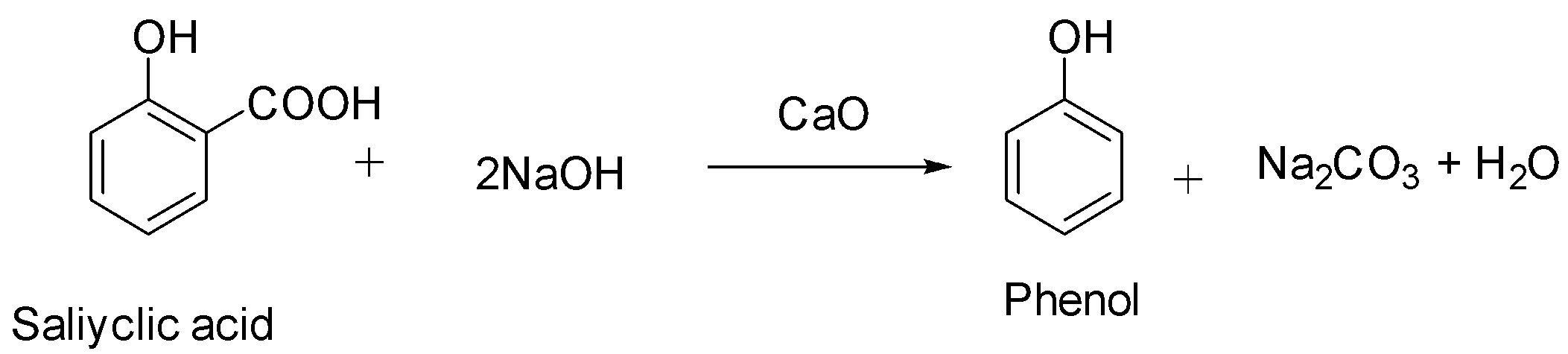
Hence, the correct option among the following is option A (salicylic acid).
Additional Information:-
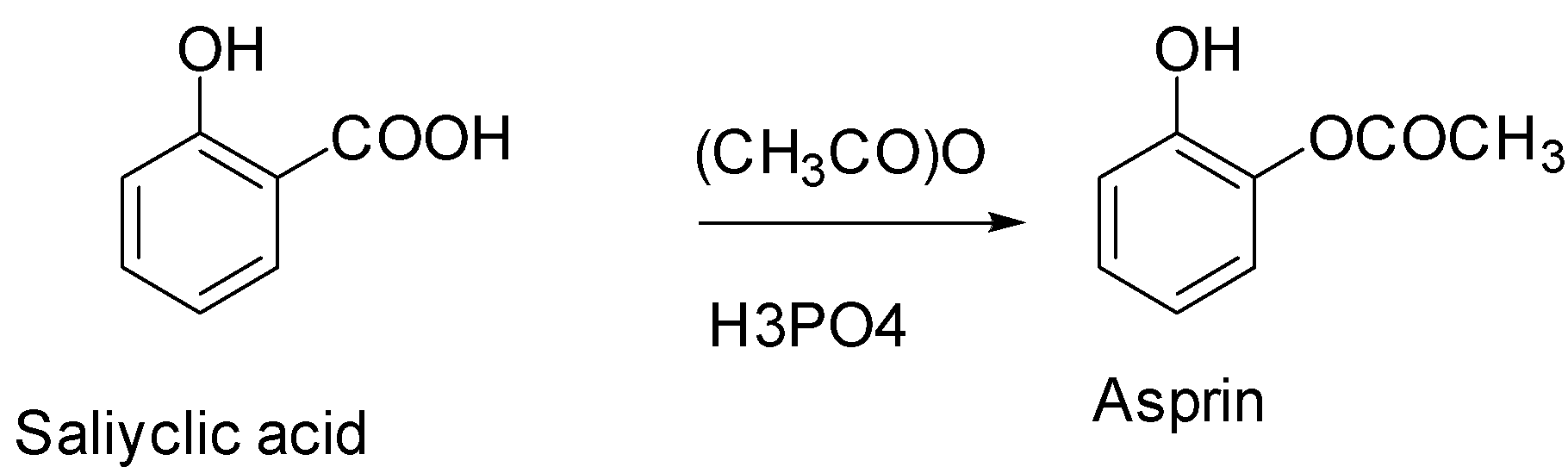
(1) Salicylic acid is a very important precursor for the preparation of aspirin. Aspirin is a drug given to a patient shortly after a heart attack as it decreases the risk of death.
(2) We can prepare aspirin by treating salicylic acid with acetic acid in the presence of an acid catalyst. The phenol group on the salicylic acid forms an ester with the carboxyl group on the acetic acid.
Note:
(1) Decarboxylation is a chemical process that involves the removal of carboxyl groups and releases carbon dioxide from the reaction system. Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain.
(2) Decarboxylation of sodium salts of carboxylic acids by using soda lime to form alkanes is known as the Duma reaction.
(3) The mixture of calcium hydroxide and sodium or potassium hydroxide (soda lime), both are corrosive materials. It is corrosive to metals and tissue. Hence, should be handled carefully.

Only salicylic acid (2-Hydroxybenzoic acid) on decarboxylation gives phenol.

Complete answer:
We must know that salicylic acid will give phenol with \[{\text{CaO}}\] and \[{\text{NaOH}}\]. The mixture of \[{\text{CaO}}\] and \[{\text{NaOH}}\] is called soda lime. When we heat carboxylic acids with soda lime, they undergo decarboxylation. The by-products in this reaction apart from phenol are sodium carbonate and water.

Hence, the correct option among the following is option A (salicylic acid).
Additional Information:-
(1) Salicylic acid is a very important precursor for the preparation of aspirin. Aspirin is a drug given to a patient shortly after a heart attack as it decreases the risk of death.
(2) We can prepare aspirin by treating salicylic acid with acetic acid in the presence of an acid catalyst. The phenol group on the salicylic acid forms an ester with the carboxyl group on the acetic acid.

Note:
(1) Decarboxylation is a chemical process that involves the removal of carboxyl groups and releases carbon dioxide from the reaction system. Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain.
(2) Decarboxylation of sodium salts of carboxylic acids by using soda lime to form alkanes is known as the Duma reaction.
(3) The mixture of calcium hydroxide and sodium or potassium hydroxide (soda lime), both are corrosive materials. It is corrosive to metals and tissue. Hence, should be handled carefully.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




