
Which of the following undergoes nucleophilic substitution by ${S_N}1$ mechanism at fastest rate:
A. Ethyl chloride
B.Isopropyl chloride
C.Benzyl chloride
D.Chloro benzene
Answer
575.4k+ views
Hint:As we know that ${S_N}1$ is an unimolecular reaction. In ${S_N}1$ reaction the intermediate formed is a carbocation. We will have to analyse the stability of the carbocation formed to get an idea about the rate of the reaction.
Complete step by step answer:
We know that ${S_N}1$ reaction has carbocation as the intermediate. The compound with most stable carbocation will undergo the fastest ${S_N}1$ mechanism. The electron donating groups stabilize the carbocation. That means resonance, inductive effect and hyperconjugation makes the carbocation stable. Ethyl chloride, isopropyl chloride and chloro benzene cannot undergo resonance . But in benzyl chloride , when the chlorine leaves via nucleophilic substitution by ${S_N}1$ mechanism , the carbocation formed stabilises because it undergoes resonance with the benzene ring which act as an electron group. So it automatically stabilizes the carbocation. The most stable carbocation will undergo the fastest nucleophilic substitution by ${S_N}1$ mechanism.
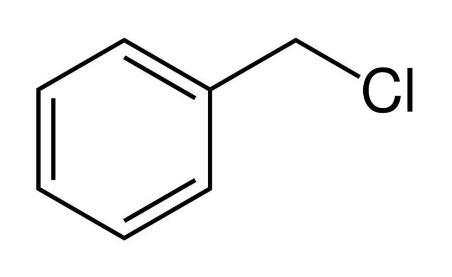
So from the above explanation it is clear to us that the correct option of the given question is C Benzyl chloride.
Additional information:
The order of ${S_N}1$ mechanism is $3^\circ > 2^\circ > 1^\circ $ , that is tertiary $ > $secondary$ > $ primary. ${S_N}1$ mechanism is preferred in polar protic solvent. ${S_N}2$ mechanism is preferred in polar aprotic solvent.
Note:
Always remember that in ${S_N}1$ mechanism we check the stability of the carbocation formed. The electron donating groups stabilize the carbocation thus it decides the rate of the ${S_N}1$ mechanism. ${S_N}1$ mechanism is preferred in polar protic solvent. Resonance stabilizes the carbocation formed in the nucleophilic substitution by ${S_N}1$ mechanism in the benzyl chloride.
Complete step by step answer:
We know that ${S_N}1$ reaction has carbocation as the intermediate. The compound with most stable carbocation will undergo the fastest ${S_N}1$ mechanism. The electron donating groups stabilize the carbocation. That means resonance, inductive effect and hyperconjugation makes the carbocation stable. Ethyl chloride, isopropyl chloride and chloro benzene cannot undergo resonance . But in benzyl chloride , when the chlorine leaves via nucleophilic substitution by ${S_N}1$ mechanism , the carbocation formed stabilises because it undergoes resonance with the benzene ring which act as an electron group. So it automatically stabilizes the carbocation. The most stable carbocation will undergo the fastest nucleophilic substitution by ${S_N}1$ mechanism.

So from the above explanation it is clear to us that the correct option of the given question is C Benzyl chloride.
Additional information:
The order of ${S_N}1$ mechanism is $3^\circ > 2^\circ > 1^\circ $ , that is tertiary $ > $secondary$ > $ primary. ${S_N}1$ mechanism is preferred in polar protic solvent. ${S_N}2$ mechanism is preferred in polar aprotic solvent.
Note:
Always remember that in ${S_N}1$ mechanism we check the stability of the carbocation formed. The electron donating groups stabilize the carbocation thus it decides the rate of the ${S_N}1$ mechanism. ${S_N}1$ mechanism is preferred in polar protic solvent. Resonance stabilizes the carbocation formed in the nucleophilic substitution by ${S_N}1$ mechanism in the benzyl chloride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




