
Which of the following tests is suitable to differentiate between aniline and benzylamine?
A. Aniline gives dye test on diazotization and reaction with $\beta - naphthol$ while benzylamine gives alcohol.
B. Benzylamine gives green dry with $\beta - naphthol$ after diazotization while aniline gives orange dye.
C. Aniline gives a carbylamine reaction while benzylamine does not.
D. Aniline gives a carbylamine reaction while benzylamine does not.
Answer
569.1k+ views
Hint: We have to remember that the aniline (IUPAC name- Benzeneamine) is an organic compound, chemical formula is \[{C_6}{H_5}N{H_2}\]. It belongs to phenylamines. It is the simplest amine in the aromatic amines. It is oily liquid and colourless. Benzylamine (IUPAC name-\[1 - Phenylmethanamine\]) is a colourless water soluble liquid. It is used in the pharmaceutical industry..
Complete step by step answer:
We must remember that the amino groups are basic in nature and these are easily reacted with acids to form salts. That salts are soluble in water. Benzylamine and aniline are distinguished by the reaction with the help of nitrous acid $(HN{O_2})$. Nitrous acid is prepared from hydrogen chloride $\left( {HCl} \right)$ and sodium nitrite $\left( {NaN{O_2}} \right)$.
We can write the chemical equation for the reaction of benzylamine with nitrous acid as,
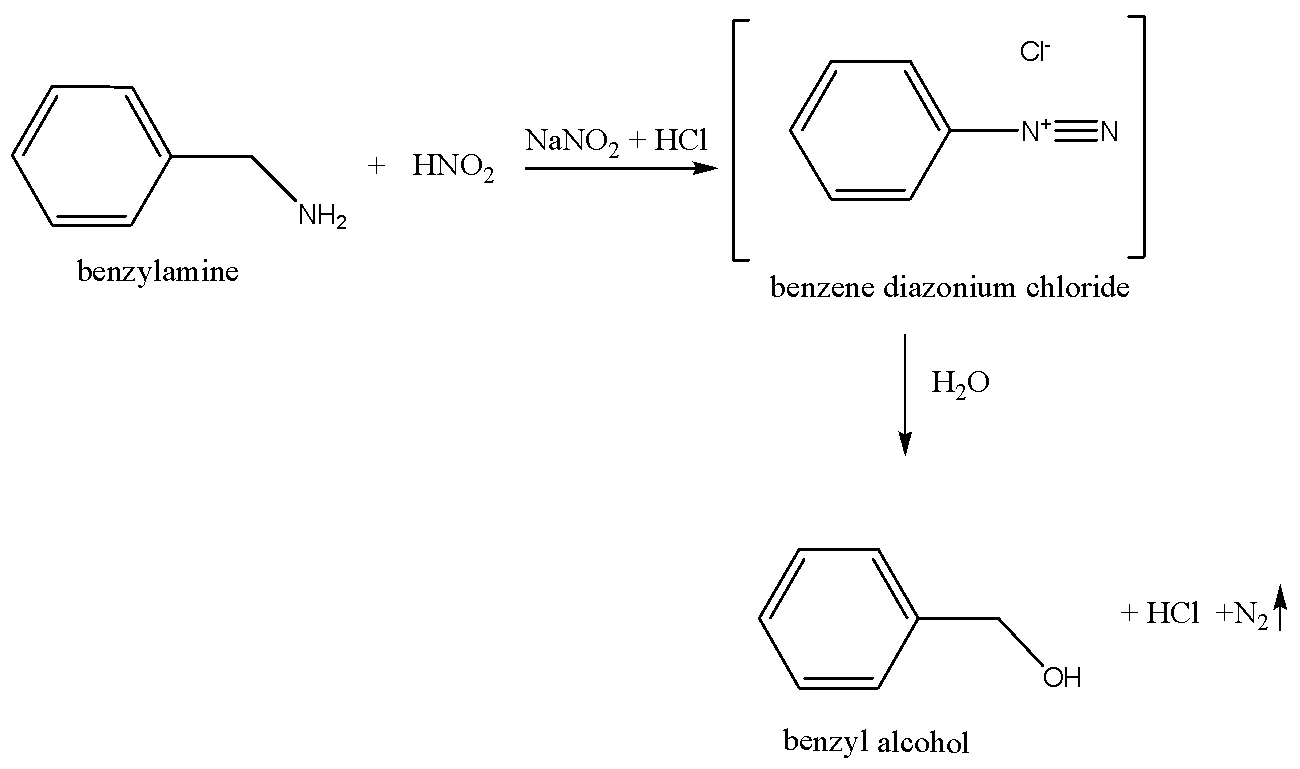
Benzylamine reacts with nitrous acid (generated in situ) to give benzene diazonium salt, which is unstable and then aqueous solution of benzene diazonium salt gives benzyl alcohol and hydrochloric acid and nitrogen is evolved.
Aniline reaction with nitrous acid
Primary amine reaction with nitrous acid is also known as azo dye test. The chemical reaction as,
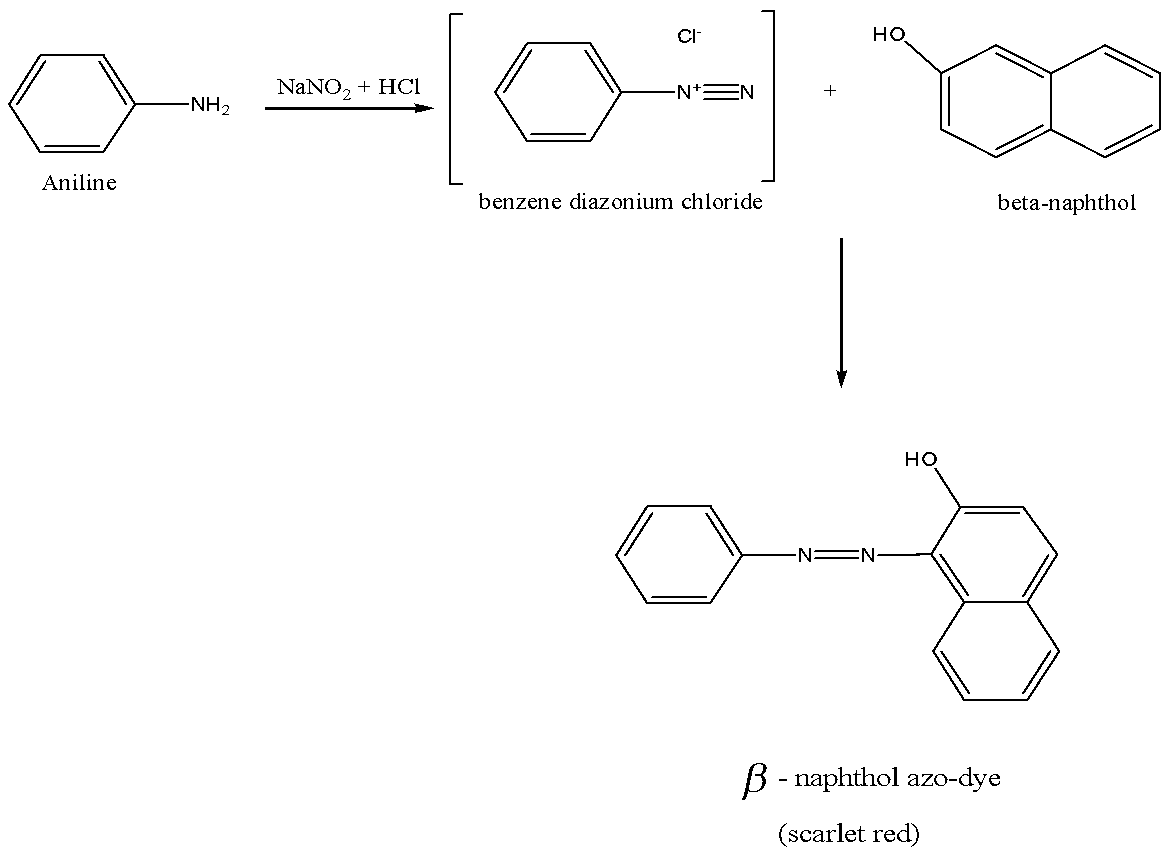
We must remember that the aniline react with nitrous acid to give benzene diazonium chloride, which is reacted with $\beta - naphthol$(in alkaline solution) to give beta-naphthol azo dye, which scarlet red in colour and the dye is sparingly soluble in water.
From the above reactions, Aniline gives dye test on diazotization and reaction with $\beta - naphthol$ while benzylamine gives alcohol.
Option A. Aniline gives dye test on diazotization and reaction with $\beta - naphthol$ while benzylamine gives alcohol is the correct answer.
So, the correct answer is Option A.
Note: We have to remember that in the azo dye test reaction add diazonium ion into the alkaline solution of $\beta - naphthol$in always. During diazotization, maintain the temperature below ${5^0}C$, because diazonium chloride is unstable. Aniline is used largely in the preparation of methylenedianiline. And also used in rubber industries. We must remember that the primary $({1^0})$amine is one hydrogen atom of ammonia that is replaced by alkyl or aryl groups. For example $C{H_3}N{H_2}$, methyl amine and ${C_6}{H_5}N{H_2}$, aniline. Primary aromatic amines are used for manufacture of azo dyes. Coloured azo-compounds are widely used in dyeing industries. One of the examples is Methyl orange.
Complete step by step answer:
We must remember that the amino groups are basic in nature and these are easily reacted with acids to form salts. That salts are soluble in water. Benzylamine and aniline are distinguished by the reaction with the help of nitrous acid $(HN{O_2})$. Nitrous acid is prepared from hydrogen chloride $\left( {HCl} \right)$ and sodium nitrite $\left( {NaN{O_2}} \right)$.
We can write the chemical equation for the reaction of benzylamine with nitrous acid as,

Benzylamine reacts with nitrous acid (generated in situ) to give benzene diazonium salt, which is unstable and then aqueous solution of benzene diazonium salt gives benzyl alcohol and hydrochloric acid and nitrogen is evolved.
Aniline reaction with nitrous acid
Primary amine reaction with nitrous acid is also known as azo dye test. The chemical reaction as,

We must remember that the aniline react with nitrous acid to give benzene diazonium chloride, which is reacted with $\beta - naphthol$(in alkaline solution) to give beta-naphthol azo dye, which scarlet red in colour and the dye is sparingly soluble in water.
From the above reactions, Aniline gives dye test on diazotization and reaction with $\beta - naphthol$ while benzylamine gives alcohol.
Option A. Aniline gives dye test on diazotization and reaction with $\beta - naphthol$ while benzylamine gives alcohol is the correct answer.
So, the correct answer is Option A.
Note: We have to remember that in the azo dye test reaction add diazonium ion into the alkaline solution of $\beta - naphthol$in always. During diazotization, maintain the temperature below ${5^0}C$, because diazonium chloride is unstable. Aniline is used largely in the preparation of methylenedianiline. And also used in rubber industries. We must remember that the primary $({1^0})$amine is one hydrogen atom of ammonia that is replaced by alkyl or aryl groups. For example $C{H_3}N{H_2}$, methyl amine and ${C_6}{H_5}N{H_2}$, aniline. Primary aromatic amines are used for manufacture of azo dyes. Coloured azo-compounds are widely used in dyeing industries. One of the examples is Methyl orange.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




