
Which of the following substances is antiferromagnetic?
(a).$MnO$
(b).$Cr{{O}_{2}}$
(c).$F{{e}_{3}}{{O}_{4}}$
(d).$NaCl$
Answer
592.2k+ views
Hint: In materials that display antiferromagnetism, atom or molecular magnetic moments, typically associated with electron spins, align in a normal pattern with adjacent spins (on different sublattices) pointing in opposite directions.
Complete answer:
One component of the combined electromagnetic force is magnetism. It refers to physical effects induced by the force of magnets, objects creating fields that attract or repel other objects.
A magnetic domain is a region within a magnetic material where a uniform direction of magnetization is in. This implies the individual atomic magnetic moments are associated with each other and point in the same direction.
These are the products which are ferromagnetic, ferrimagnetic, and antiferromagnetic. There are no magnetic domains in paramagnetic and diamagnetic materials, in which the dipoles align in response to an external field but do not align spontaneously.
The$MnO$ has antiferromagnetism..
Anti-Ferromagnetism is a form of solid magnetism in which adjacent ions act as a tiny magnet. At low temperature, some ions align themselves.
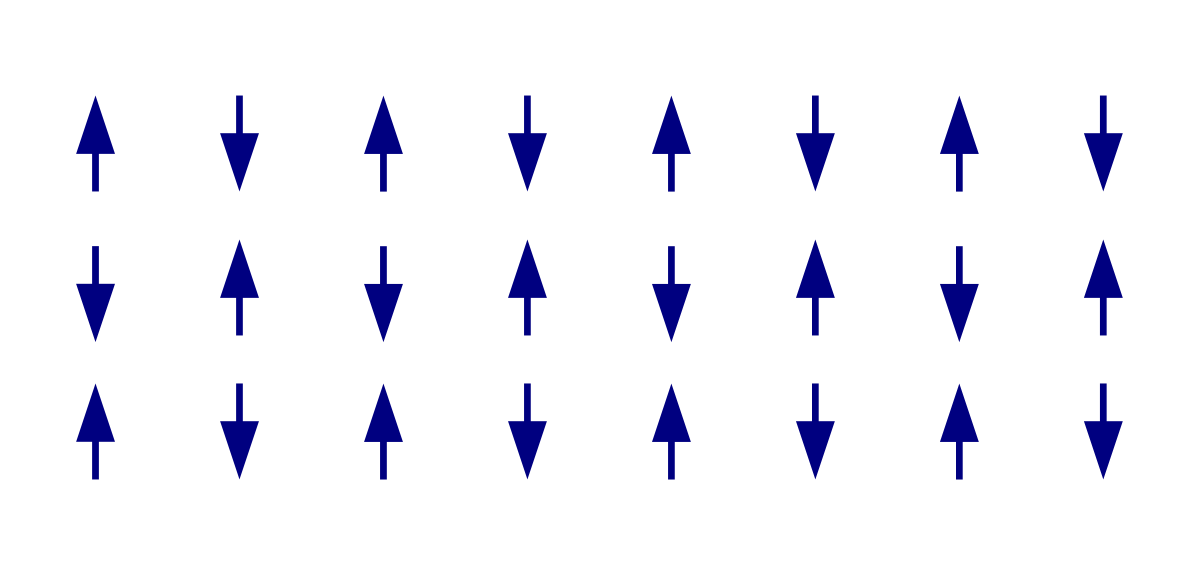
Adjacent ions that serve as small magnets (in this case manganese ions, $M{{n}^{2+}}$) bind themselves spontaneously to the opposite or antiparallel at relatively low temperatures.
$NaCl$ has a diamagnetic character. Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates in them in the opposite direction an induced magnetic field which causes repulsive force.
$Cr{{O}_{2}}$ has ferromagnetic properties. Ferromagnetism is the fundamental process by which certain materials shape, or are attracted to, permanent magnets.
$F{{e}_{3}}{{O}_{4}}$is ferrimagnetic at room temperature. Ferrimagnetic material is one that has populations of atoms with opposing magnetic moments, as in antiferromagnetism; however, the opposing moments are different in ferrimagnetic materials and there is a spontaneous magnetization.
Note:
-The root of magnetism lies in the electrons' orbital and spin motions, and how they interact with each other.
-Some may find that shocking, but all the matter is magnetic. It is just because some materials are much more magnetic than others. The principal difference is that there is no mutual interaction of atomic magnetic moments in some materials, while there is a very strong interaction between atomic moments in other materials.
Complete answer:
One component of the combined electromagnetic force is magnetism. It refers to physical effects induced by the force of magnets, objects creating fields that attract or repel other objects.
A magnetic domain is a region within a magnetic material where a uniform direction of magnetization is in. This implies the individual atomic magnetic moments are associated with each other and point in the same direction.
These are the products which are ferromagnetic, ferrimagnetic, and antiferromagnetic. There are no magnetic domains in paramagnetic and diamagnetic materials, in which the dipoles align in response to an external field but do not align spontaneously.
The$MnO$ has antiferromagnetism..
Anti-Ferromagnetism is a form of solid magnetism in which adjacent ions act as a tiny magnet. At low temperature, some ions align themselves.

Adjacent ions that serve as small magnets (in this case manganese ions, $M{{n}^{2+}}$) bind themselves spontaneously to the opposite or antiparallel at relatively low temperatures.
$NaCl$ has a diamagnetic character. Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates in them in the opposite direction an induced magnetic field which causes repulsive force.
$Cr{{O}_{2}}$ has ferromagnetic properties. Ferromagnetism is the fundamental process by which certain materials shape, or are attracted to, permanent magnets.
$F{{e}_{3}}{{O}_{4}}$is ferrimagnetic at room temperature. Ferrimagnetic material is one that has populations of atoms with opposing magnetic moments, as in antiferromagnetism; however, the opposing moments are different in ferrimagnetic materials and there is a spontaneous magnetization.
Note:
-The root of magnetism lies in the electrons' orbital and spin motions, and how they interact with each other.
-Some may find that shocking, but all the matter is magnetic. It is just because some materials are much more magnetic than others. The principal difference is that there is no mutual interaction of atomic magnetic moments in some materials, while there is a very strong interaction between atomic moments in other materials.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




