
Which of the following structures represents the neoprene polymer?
1)
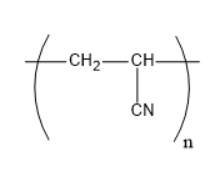
2)
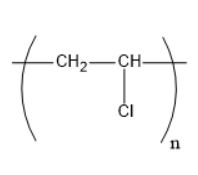
3)
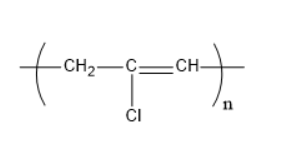
4)
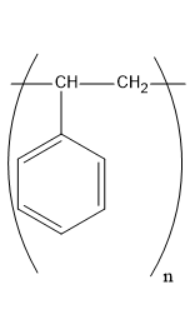




Answer
558.6k+ views
Hint: Neoprene (CR), likewise called polychloroprene or chloroprene elastic, manufactured elastic created by the polymerization (or connecting together of single atoms into large, different unit particles) of chloroprene. We basically call it synthetic rubber in daily-life. Neoprene shows great chemical stability and keeps up adaptability over a wide temperature range.
Complete solution:
A decent broadly useful elastic, neoprene is esteemed for its high rigidity, versatility, oil and fire opposition, and protection from debasement by oxygen and ozone; nonetheless, its significant expense restricts its utilization to unique properties applications.
The structure of neoprene is:
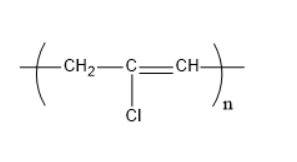
One of the primary effective manufactured rubbers, polychloroprene was first set up in 1930 by Arnold Collins, an American scientific expert in Wallace Hume Carothers' examination bunch at E.I. du Pont de Nemours and Company (presently DuPont Company), while exploring results of divinylacetylene. DuPont promoted the material as Neoprene, a reserved name that has since gotten nonexclusive.
It was once set up by treating acetylene with cuprous chloride structure monovinyl acetylene, which was treated thusly with hydrochloric corrosive to yield chloroprene. In present-day creation, it is gotten by the chlorination of butadiene or isoprene. To deal with chloroprene into elastic, it is emulsified in water and afterward polymerized through the activity of free-extremist initiators. In the resultant polymer chain, the chloroprene rehashing unit can embrace various structures; the most widely recognized is trans-polychloroprene.
Hence,the correct option is C.
Note: Chloroprene (also known as 2-chlorobutadiene) is a colourless, toxic, flammable liquid. This polymer will in general crystallize and solidify gradually at temperatures beneath around \[10^\circ C\](\[50^\circ F\]). It additionally solidifies on extending, so restored segments are solid even without the option of fillers, for example, carbon dark. Since the double-bond connection between the carbon atoms is protected by the pendant particles and CH2 groups, the sub-atomic interlinking fundamental for vulcanizing the polymer to a relieved elastic is normally affected through the chlorine molecule.
Complete solution:
A decent broadly useful elastic, neoprene is esteemed for its high rigidity, versatility, oil and fire opposition, and protection from debasement by oxygen and ozone; nonetheless, its significant expense restricts its utilization to unique properties applications.
The structure of neoprene is:

One of the primary effective manufactured rubbers, polychloroprene was first set up in 1930 by Arnold Collins, an American scientific expert in Wallace Hume Carothers' examination bunch at E.I. du Pont de Nemours and Company (presently DuPont Company), while exploring results of divinylacetylene. DuPont promoted the material as Neoprene, a reserved name that has since gotten nonexclusive.
It was once set up by treating acetylene with cuprous chloride structure monovinyl acetylene, which was treated thusly with hydrochloric corrosive to yield chloroprene. In present-day creation, it is gotten by the chlorination of butadiene or isoprene. To deal with chloroprene into elastic, it is emulsified in water and afterward polymerized through the activity of free-extremist initiators. In the resultant polymer chain, the chloroprene rehashing unit can embrace various structures; the most widely recognized is trans-polychloroprene.
Hence,the correct option is C.
Note: Chloroprene (also known as 2-chlorobutadiene) is a colourless, toxic, flammable liquid. This polymer will in general crystallize and solidify gradually at temperatures beneath around \[10^\circ C\](\[50^\circ F\]). It additionally solidifies on extending, so restored segments are solid even without the option of fillers, for example, carbon dark. Since the double-bond connection between the carbon atoms is protected by the pendant particles and CH2 groups, the sub-atomic interlinking fundamental for vulcanizing the polymer to a relieved elastic is normally affected through the chlorine molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




