
Which of the following statements is/are not false? (This question has multiple correct answers).
(a)- Sorbitol and mannitol are epimers
(b)- Sorbitol and mannitol do not show the relationship as an object and its mirror image
(c)- Sorbitol and mannitol differ in the number of –OH group in the molecule
(d)- All of the above
Answer
513k+ views
Hint: Both sorbitol and mannitol are carbohydrates in which there are six carbon atoms. epimers are those compounds if they differ in configuration only at one carbon atom.
Complete answer:
Sorbitol and Mannitol are both carbohydrates because they are made up of carbon, hydrogen, and oxygen atoms. The formula of both sorbitol and mannitol are the same, and its formula is ${{C}_{6}}{{H}_{14}}{{O}_{6}}$.
Since the formula of sorbitol and mannitol are the same but the arrangements of the groups around the carbon atoms are different. The structures of them are given below:
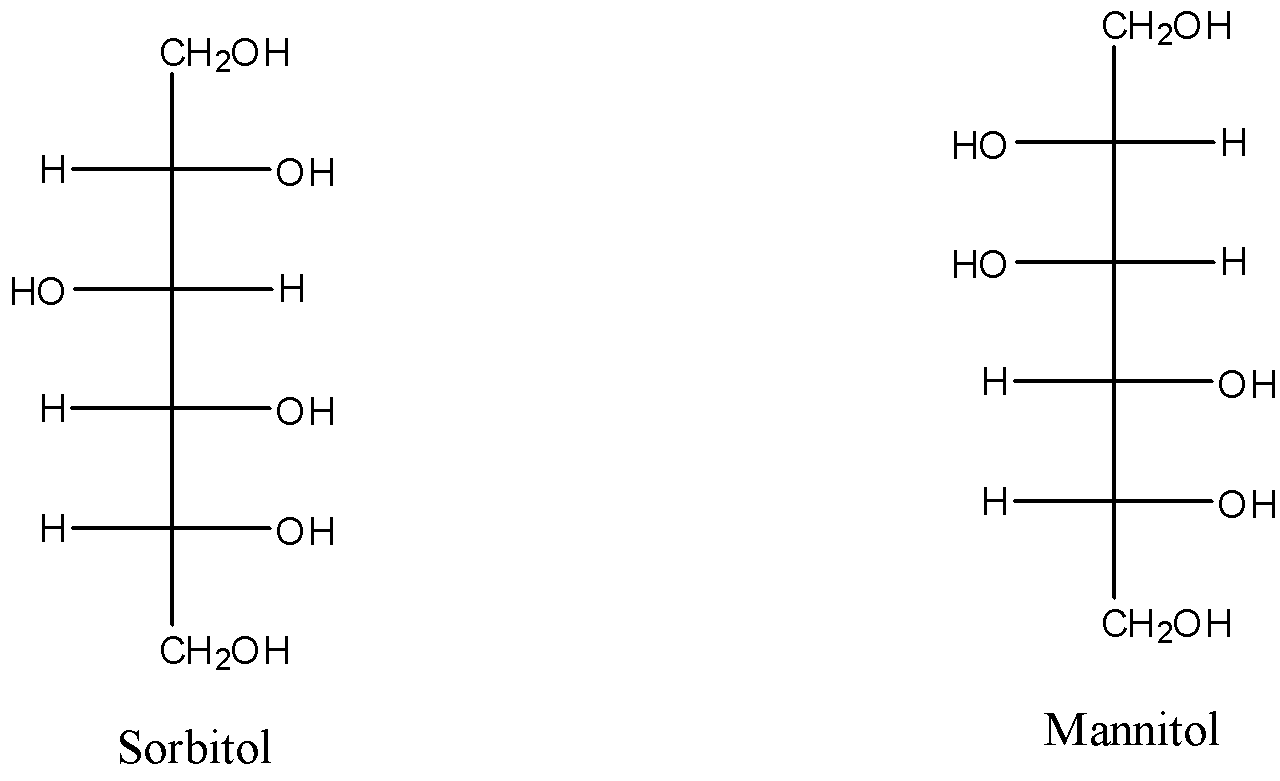
As we can see that there is only one change in the configuration at one carbon atom. So, they are epimers. Therefore, statement (a) is correct.
The relationship between an item and its reflection, since the configuration is uniform to one single carbon atom, cannot be shown in Sorbitol and mannitol. So, statement (b) is also correct.
Both sorbitol and mannitol have six hydroxyl groups (-OH), only the arrangements of the groups are different. So, statement (c) is incorrect.
Therefore, from the given options, option (a) and option (b) are correct answers.
Note:
Both sorbitol and mannitol are sugar alcohol and are isomers of each other. Their properties like uses, melting points, and sources are different even though they look similar.
Complete answer:
Sorbitol and Mannitol are both carbohydrates because they are made up of carbon, hydrogen, and oxygen atoms. The formula of both sorbitol and mannitol are the same, and its formula is ${{C}_{6}}{{H}_{14}}{{O}_{6}}$.
Since the formula of sorbitol and mannitol are the same but the arrangements of the groups around the carbon atoms are different. The structures of them are given below:

As we can see that there is only one change in the configuration at one carbon atom. So, they are epimers. Therefore, statement (a) is correct.
The relationship between an item and its reflection, since the configuration is uniform to one single carbon atom, cannot be shown in Sorbitol and mannitol. So, statement (b) is also correct.
Both sorbitol and mannitol have six hydroxyl groups (-OH), only the arrangements of the groups are different. So, statement (c) is incorrect.
Therefore, from the given options, option (a) and option (b) are correct answers.
Note:
Both sorbitol and mannitol are sugar alcohol and are isomers of each other. Their properties like uses, melting points, and sources are different even though they look similar.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




