
Which of the following statements is/are correct about tetrahedral voids (TVs) in an FCC unit cell? (This question has multiple correct options)
a.) Number of TVs per atom in FCC unit cells is 2.
b.) Number of TVs per unit cell is 8.
c.) Number of TVs is twice the number of atoms in the FCC unit cell.
d.) Number of TVs is equal to the number of atoms in the FCC unit cell.
Answer
588.6k+ views
Hint: To tell the correct statement, we know that in hexagonal close packing (HCP) and cubic close packing (CCP) when sphere of second layer is above the void of first layer(or vice versa), tetrahedral void is formed.
Complete step by step answer:
To answer this question, we should first know that void means that it is empty space. We should first observe the hexagonal close packing and cubic close packing; we came to know that each sphere of second layer touches with three spheres of first layer. And by this, it leaves a small space in between which is known as a tetrahedral site and this space between four touching spheres is called a tetrahedral void. We note that a sphere touches three spheres in the below layer and three spheres in the above layer, and then we came to know that there are two tetrahedral sites associated with one sphere.
Now, we will check the statements that are given in question, to check whether the statement above the tetrahedral void is correct or not.
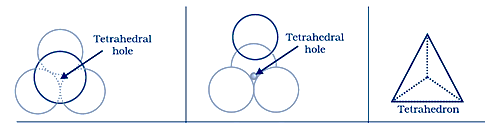
By looking at the structure of FCC, we came to know that one corner and its face centres form a tetrahedral void. We should note that, in FCC, two tetrahedral voids are obtained along one cube diagonal. So, in FCC, 8 tetrahedral voids are present. In FCC, the total number of tetrahedral voids is equal to eight. So, the correct answer is “Option A,B and C”.
Note: We should also know about octahedral voids. At other places, the triangular voids in the second layer are above the triangular voids in the first layer, then the octahedral void is formed. The apex of both the triangles point in opposite directions upward and downward.
Complete step by step answer:
To answer this question, we should first know that void means that it is empty space. We should first observe the hexagonal close packing and cubic close packing; we came to know that each sphere of second layer touches with three spheres of first layer. And by this, it leaves a small space in between which is known as a tetrahedral site and this space between four touching spheres is called a tetrahedral void. We note that a sphere touches three spheres in the below layer and three spheres in the above layer, and then we came to know that there are two tetrahedral sites associated with one sphere.
Now, we will check the statements that are given in question, to check whether the statement above the tetrahedral void is correct or not.

By looking at the structure of FCC, we came to know that one corner and its face centres form a tetrahedral void. We should note that, in FCC, two tetrahedral voids are obtained along one cube diagonal. So, in FCC, 8 tetrahedral voids are present. In FCC, the total number of tetrahedral voids is equal to eight. So, the correct answer is “Option A,B and C”.
Note: We should also know about octahedral voids. At other places, the triangular voids in the second layer are above the triangular voids in the first layer, then the octahedral void is formed. The apex of both the triangles point in opposite directions upward and downward.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE

Calculate the equivalent resistance between a and b class 12 physics CBSE

How many states of matter are there in total class 12 chemistry CBSE

Which of the following is the best conductor of electricity class 12 physics CBSE




