
Which of the following statements is wrong?
(This question has more than one correct options)
A. The stability of hydrides increases from \[N{{H}_{3}}\] to \[Bi{{H}_{3}}\], in
group 15 of the modern periodic table
B. Nitrogen cannot form \[d\pi -p\pi \] bond.
C. Single \[N-N\] bond is weaker than single \[P-P\] bond.
D. \[{{N}_{2}}{{O}_{4}}\] has two resonating structures.
Answer
578.4k+ views
Hint: We can go option by option to solve this question. We can keep following things in mind while solving this question and they are:
As we move down the group 15 for hydrides there is a decrease in their bond dissociation enthalpy. There should be a low lying orbital present for overlap as overlapping orbitals should have similar energy and proper orientation.
Complete step by step answer:
A.The stability of hydrides increases from \[N{{H}_{3}}\] to \[Bi{{H}_{3}}\], in group 15 of the modern periodic table. This statement is wrong as thermal stability of the hydrides decreases as we go down the group in the periodic table for group 15 which is the Nitrogen family because of the increasing size. When there is a lot of difference in sizes of covalently bonded atoms the bonding gets weaker.
B.Nitrogen cannot form \[d\pi -p\pi \] bond. This statement is correct as nitrogen does not have low lying or low energy d orbitals to participate in bonding so it does not form \[d\pi -p\pi \] bond.
C.Single \[N-N\] bond is weaker than a single P-P bond. The N-N Sigma bond is weaker than the \[P-P\] sigma bond due to the small bond length between the nitrogen atoms. The non-bonding electrons (lone pair of electrons) of both the atoms repel each other making it weaker than P-P sigma bond.
D.\[{{N}_{2}}{{O}_{4}}\] has two resonating structures. This statement is incorrect. There are in total four stable resonance structures for \[{{N}_{2}}{{O}_{4}}\].
Hence, the correct answer is option B and C.
Note:
The four resonating structures of \[{{N}_{2}}{{O}_{4}}\] are given below:
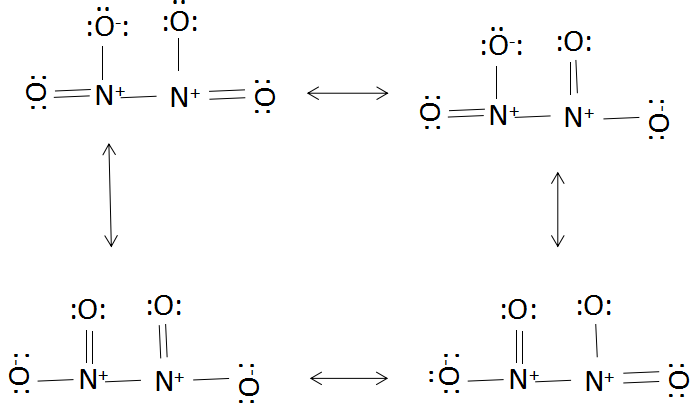
As we move down the group 15 for hydrides there is a decrease in their bond dissociation enthalpy. There should be a low lying orbital present for overlap as overlapping orbitals should have similar energy and proper orientation.
Complete step by step answer:
A.The stability of hydrides increases from \[N{{H}_{3}}\] to \[Bi{{H}_{3}}\], in group 15 of the modern periodic table. This statement is wrong as thermal stability of the hydrides decreases as we go down the group in the periodic table for group 15 which is the Nitrogen family because of the increasing size. When there is a lot of difference in sizes of covalently bonded atoms the bonding gets weaker.
B.Nitrogen cannot form \[d\pi -p\pi \] bond. This statement is correct as nitrogen does not have low lying or low energy d orbitals to participate in bonding so it does not form \[d\pi -p\pi \] bond.
C.Single \[N-N\] bond is weaker than a single P-P bond. The N-N Sigma bond is weaker than the \[P-P\] sigma bond due to the small bond length between the nitrogen atoms. The non-bonding electrons (lone pair of electrons) of both the atoms repel each other making it weaker than P-P sigma bond.
D.\[{{N}_{2}}{{O}_{4}}\] has two resonating structures. This statement is incorrect. There are in total four stable resonance structures for \[{{N}_{2}}{{O}_{4}}\].
Hence, the correct answer is option B and C.
Note:
The four resonating structures of \[{{N}_{2}}{{O}_{4}}\] are given below:
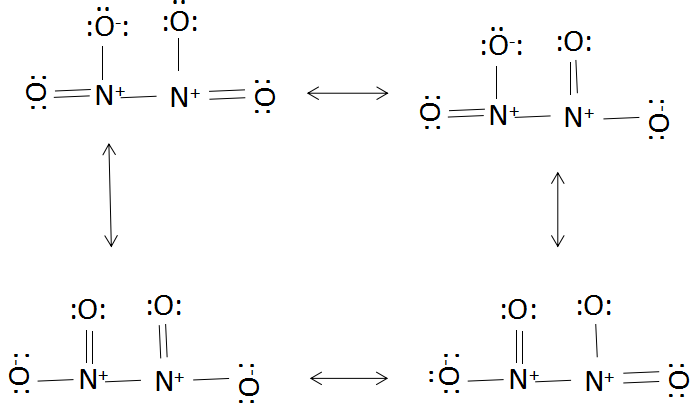
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




