
Which of the following statements is wrong about chloroform?
A.Chloroform is an anesthetic.
B.Chloroform has a distorted tetrahedral shape.
C.Chloroform is used as a solvent.
D.Chloroform has $s{p^2}$ hybridized carbon atom.
Answer
582.3k+ views
Hint: Chloroform is a poly-halogen compound. It is a colorless liquid with a characteristic order. It is formed by the reaction of chlorine in excess with methane in which water is the by-product.
Complete step by step answer:
Chloroform has the chemical formula $CHC{l_3}$ in which three chlorine atoms and one hydrogen atom are attached to the carbon atom. It is also called trichloromethane. Its structure is given as-
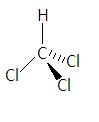
In this structure-
-The single bond represents that the bonds are in the plane of paper.
-The heavy line represents that the bond is above the plane of paper towards the observer
-And the dashed line represents that the bond below the plane of paper means away from the observer.
- Here the carbon is $s{p^3}$ hybridized because the electrons in s-orbital and p-orbital combine to form new $s{p^3}$ hybridized orbitals.
-Here the structure should be tetrahedral but due to the difference of electro-negativity between the atoms, the shape becomes distorted tetrahedral as chlorine molecules try to have maximum distance from each other due to electron-electron repulsion.
-The uses of chloroform are-
1.It is used as an anesthetic.
2.It is used as a solvent and dry cleaning agent.
3.It is also used in manufacture of liquid refrigerant and PTFE plastics.
4.It is used in production of synthetic rubber.
So the statement that Chloroform has $s{p^2}$ hybridized carbon atoms is wrong.
Hence the correct answer is D.
Note:
The properties of chloroform are-
1.It is a clear and colourless liquid.
2.It is slightly soluble in water.
3.It is denser than water.
4.It is non-flammable but can burn under extreme conditions.
Complete step by step answer:
Chloroform has the chemical formula $CHC{l_3}$ in which three chlorine atoms and one hydrogen atom are attached to the carbon atom. It is also called trichloromethane. Its structure is given as-

In this structure-
-The single bond represents that the bonds are in the plane of paper.
-The heavy line represents that the bond is above the plane of paper towards the observer
-And the dashed line represents that the bond below the plane of paper means away from the observer.
- Here the carbon is $s{p^3}$ hybridized because the electrons in s-orbital and p-orbital combine to form new $s{p^3}$ hybridized orbitals.
-Here the structure should be tetrahedral but due to the difference of electro-negativity between the atoms, the shape becomes distorted tetrahedral as chlorine molecules try to have maximum distance from each other due to electron-electron repulsion.
-The uses of chloroform are-
1.It is used as an anesthetic.
2.It is used as a solvent and dry cleaning agent.
3.It is also used in manufacture of liquid refrigerant and PTFE plastics.
4.It is used in production of synthetic rubber.
So the statement that Chloroform has $s{p^2}$ hybridized carbon atoms is wrong.
Hence the correct answer is D.
Note:
The properties of chloroform are-
1.It is a clear and colourless liquid.
2.It is slightly soluble in water.
3.It is denser than water.
4.It is non-flammable but can burn under extreme conditions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




