
Which of the following statements are correct about Nylon-6,6?
A.Nylon fibre has higher tensile strength than terylene fibres.
B.Nylon fibers have lower tensile strength than terylene fibres.
C.In nylon, there is strong intermolecular H-bonding, while in terylene there is weak dipole-dipole interaction.
D.In nylon, there is weak dipole-dipole interaction, while in terylene there is strong intermolecular H-bonding.
Answer
559.2k+ views
Hint: Both nylon and terylene are condensation polymers and are made into synthetic fibres. Nylon fibre is used to make ropes, strings of racket, parachute cloth, fishing lines, etc. while terylenes are used for making clothes, sheets, sails, etc.
Complete step-by-step solution:
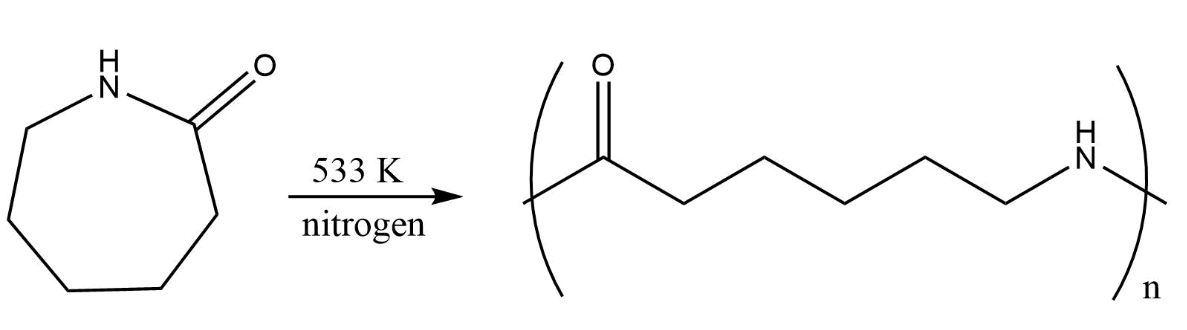
Nylon belongs to a polymer family called linear polyamides. It is made when an acidic group (COOH) on every end reacts with molecules that have amino \[(N{H_2})\] groups at each end forming strong intermolecular H-bond. Nylon 6,6 is made from hexamethylenediamine and adipic acid in exact ratio \[1:1\] acid to base. To form polymer, it is made to dry and then heated under vacuum for dehydration through the process of the condensation polymerisation reaction.
It is elastic, lustrous, very strong, damage resistant, resilient and does not absorb water or dries faster. It has high insulation and corrosion resistant. Nylon can also be synthesised from caprolactam.
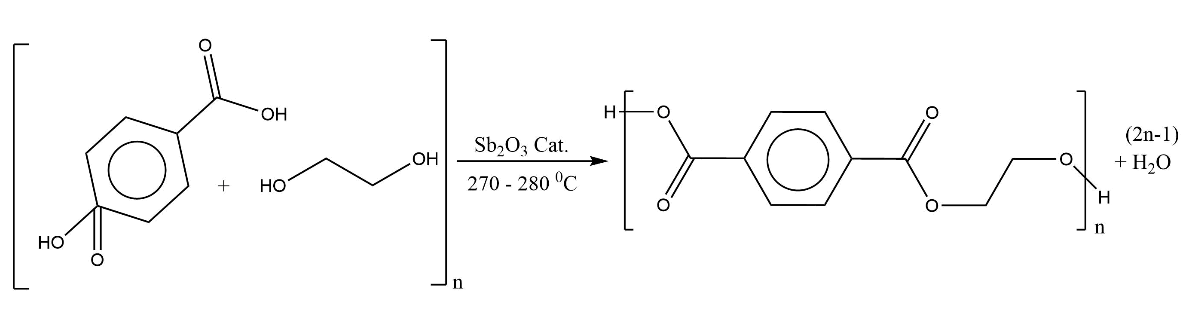
Terylene is prepared by condensation reaction between ethylene glycol and terephthalic acid. It is also known as Dacron or polyester. In condensation polymerization, when the monomers join together to form a polymer, a small molecule is lost. A polyester is made by a reaction that involves an acid with two carboxylic groups and an alcohol with two hydroxyl groups.
Here, the acid is benzene-1,4-dicarboxylic acid (terephthalic acid) and the alcohol is ethane-1,2-diol (ethylene glycol). They line up alternately and make esters with each acid group and alcohol group, losing a water molecule each time. This weakens the bonding between them and therefore they have weak dipole-dipole interactions.
Hence, the correct options are (A) and (C).
Note: In nylon 6,6, the two digits 66 indicates that the material is made from multiple monomers in combination with each other called co-monomers. Some other common variants are nylon 6, nylon \[\dfrac{6}{6}\] , etc. where the numbers indicate the number of carbon atoms between amine and acid groups.
Complete step-by-step solution:
Nylon belongs to a polymer family called linear polyamides. It is made when an acidic group (COOH) on every end reacts with molecules that have amino \[(N{H_2})\] groups at each end forming strong intermolecular H-bond. Nylon 6,6 is made from hexamethylenediamine and adipic acid in exact ratio \[1:1\] acid to base. To form polymer, it is made to dry and then heated under vacuum for dehydration through the process of the condensation polymerisation reaction.
It is elastic, lustrous, very strong, damage resistant, resilient and does not absorb water or dries faster. It has high insulation and corrosion resistant. Nylon can also be synthesised from caprolactam.

Terylene is prepared by condensation reaction between ethylene glycol and terephthalic acid. It is also known as Dacron or polyester. In condensation polymerization, when the monomers join together to form a polymer, a small molecule is lost. A polyester is made by a reaction that involves an acid with two carboxylic groups and an alcohol with two hydroxyl groups.
Here, the acid is benzene-1,4-dicarboxylic acid (terephthalic acid) and the alcohol is ethane-1,2-diol (ethylene glycol). They line up alternately and make esters with each acid group and alcohol group, losing a water molecule each time. This weakens the bonding between them and therefore they have weak dipole-dipole interactions.

Hence, the correct options are (A) and (C).
Note: In nylon 6,6, the two digits 66 indicates that the material is made from multiple monomers in combination with each other called co-monomers. Some other common variants are nylon 6, nylon \[\dfrac{6}{6}\] , etc. where the numbers indicate the number of carbon atoms between amine and acid groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




