
Which of the following statements are correct statements(s) about pyrophosphoric acid $\left( {{H_4}{P_2}{O_7}} \right)$?
A.It contains P in +5 oxidation state
B.It is dibasic acid
C.It is a reducing agent
D.it contains one P-O-P bond
Answer
590.4k+ views
Hint: $\left( {{{\rm{H}}_{\rm{4}}}{{\rm{P}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}}} \right)$ is commonly known as pyrophosphoric as well as diphosphoric acid. In pyrophosphoric acid, phosphorus is the central metal atom.
Complete step by step answer:
As the charge on hydrogen atom is +1, charge of oxygen atom is -2. Let us assume that the oxidation number of P is “x”.
So, we can calculate the value x as follows in the compound, $\left( {{H_4}{P_2}{O_7}} \right)$
$\begin{array}{l}\left( { + 1} \right) \times 4 + \left( {\rm{x}} \right) \times 2 + \left( { - 2} \right) \times 7 = 0\\{\rm{x}}\,{\rm{ = + 5}}\end{array}$
Hence the oxidation number of P in the compound $\left( {{H_4}{P_2}{O_7}} \right)$ is +5.
Therefore, option A is correct.
Structure of the compound $\left( {{H_4}{P_2}{O_7}} \right)$ as follows.
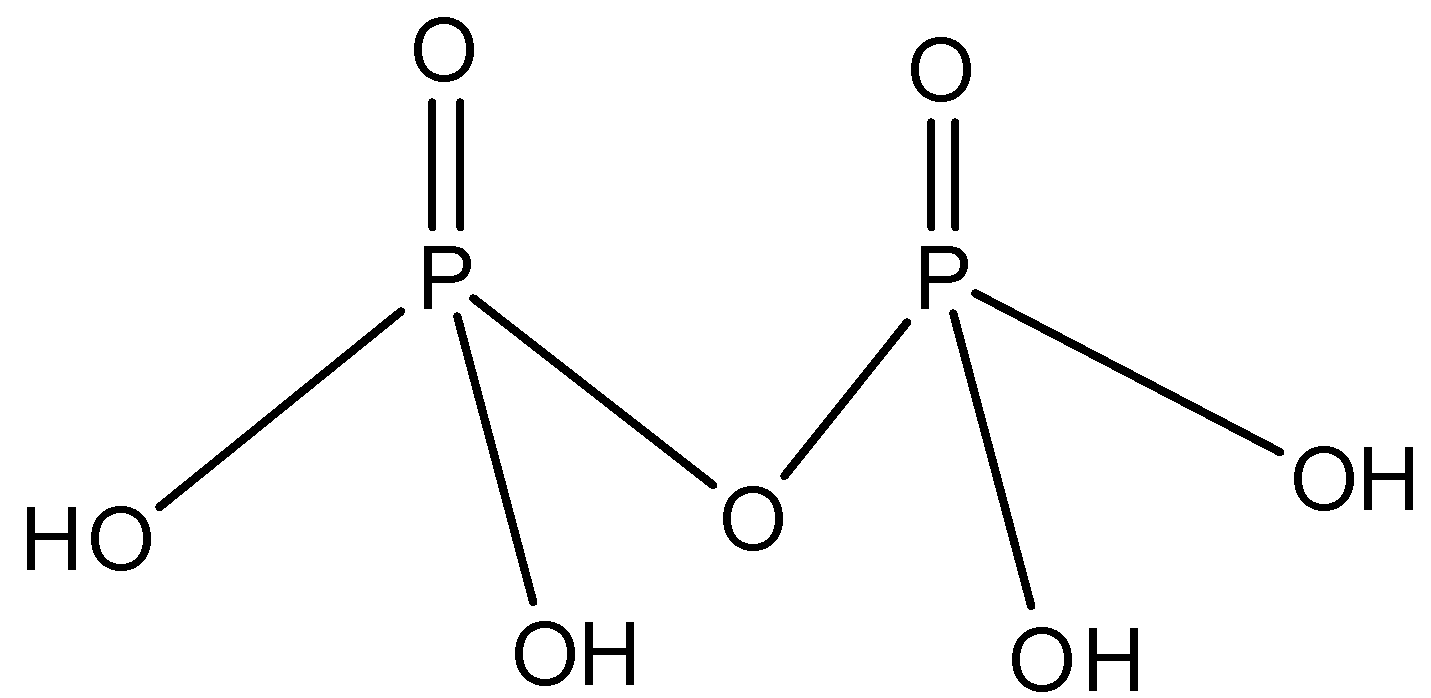
In the structure we see that the compound $\left( {{H_4}{P_2}{O_7}} \right)$ contains four -OH groups. Therefore, it is tetrabasic.
Also, in the above structure we see that there exists one P-O-P bond. Therefore, option D is correct.
From the structure, we can see that there is no P-H bond available. Hence, pyrophosphoric acid is not a reducing agent. Therefore, it is also not a reducing agent.
Therefore A and D are the correct options.
Note:
Pyrophosphoric acid is an odourless and colourless compound. It is soluble in water, ethyl alcohol and in diethyl ether.
In $\left( {{H_4}{P_2}{O_7}} \right)$, P atom has +5 oxidation state and it is a non-reducing agent due to the absence of P-H bond in it. Pyrophosphoric acid contains four -OH groups, hence it is considered to be tetrabasic compound.
Complete step by step answer:
As the charge on hydrogen atom is +1, charge of oxygen atom is -2. Let us assume that the oxidation number of P is “x”.
So, we can calculate the value x as follows in the compound, $\left( {{H_4}{P_2}{O_7}} \right)$
$\begin{array}{l}\left( { + 1} \right) \times 4 + \left( {\rm{x}} \right) \times 2 + \left( { - 2} \right) \times 7 = 0\\{\rm{x}}\,{\rm{ = + 5}}\end{array}$
Hence the oxidation number of P in the compound $\left( {{H_4}{P_2}{O_7}} \right)$ is +5.
Therefore, option A is correct.
Structure of the compound $\left( {{H_4}{P_2}{O_7}} \right)$ as follows.

In the structure we see that the compound $\left( {{H_4}{P_2}{O_7}} \right)$ contains four -OH groups. Therefore, it is tetrabasic.
Also, in the above structure we see that there exists one P-O-P bond. Therefore, option D is correct.
From the structure, we can see that there is no P-H bond available. Hence, pyrophosphoric acid is not a reducing agent. Therefore, it is also not a reducing agent.
Therefore A and D are the correct options.
Note:
Pyrophosphoric acid is an odourless and colourless compound. It is soluble in water, ethyl alcohol and in diethyl ether.
In $\left( {{H_4}{P_2}{O_7}} \right)$, P atom has +5 oxidation state and it is a non-reducing agent due to the absence of P-H bond in it. Pyrophosphoric acid contains four -OH groups, hence it is considered to be tetrabasic compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




