
Which of the following statements about ethylmethylamine is true?
A.It is a dialkylamine
B.It is a tertiary amine
C.Its IUPAC name is N-methylaminoethane
D.Its IUPAC name is N-ethylaminomethane
Answer
522.9k+ views
Hint: For finding a solution to this problem we must look at the structure of ethylmethylamine. If we know the structure then it will be easy for us to know whether it is dialkyl amine or tertiary amine. And using the structure only we can find out what is the IUPAC name of the compound. We must also look into some of its properties.
Complete answer:
Ethylmethylamine is a compound that has a chemical formula:$C{H_3}C{H_2}NHC{H_3}$. In the structure of ethylmethylamine the two groups ethane and methane are attached to amine from both sides. Following is the structure of ethylmethylamine:
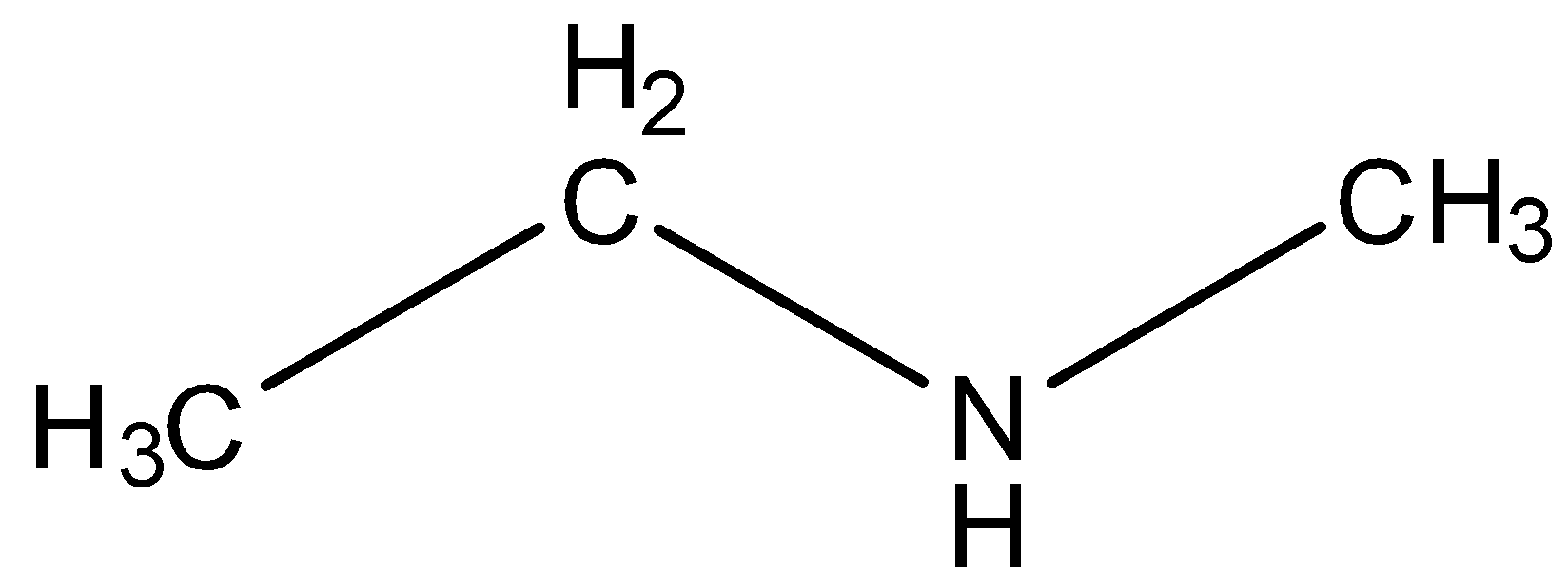
This compound is highly corrosive and highly inflammable. We can see in the structure of ethylmethylamine that there are two alkyl groups both attached on either side of amine, so it is secondary amine and not tertiary and hence it is alkylamine.
Now let us look into at the IUPAC naming of our compound:
For IUPAC naming we have to select the longest carbon chain which will be the parent group. So in ethylmethylamine the ethyl group has two carbons and the methyl group has one carbon hence ethyl becomes the parent carbon chain. Whenever amine is present with an alkyl group it is indicated by writing prefix N along with the alkyl group. Now we have methyl-substituted amine and hence the name of the compound becomes N-methylaminoethane where ethane being the parent chain.
From the above solution, it can be noted that two options match our solution.
And therefore the correct options are:
A.It is a dialkylamine
C.Its IUPAC name is N-methylaminoethane
Note:
for finding the correct solution if a structure is not known then by looking only at its name the solution of whether it is a dialkylamine or not can be found out. As it is clear that dialkylamine means amine having two alkyl groups attached to it. And for IUPAC naming just look into the parent carbon chain and to which group alkyl groups are attached.
Complete answer:
Ethylmethylamine is a compound that has a chemical formula:$C{H_3}C{H_2}NHC{H_3}$. In the structure of ethylmethylamine the two groups ethane and methane are attached to amine from both sides. Following is the structure of ethylmethylamine:

This compound is highly corrosive and highly inflammable. We can see in the structure of ethylmethylamine that there are two alkyl groups both attached on either side of amine, so it is secondary amine and not tertiary and hence it is alkylamine.
Now let us look into at the IUPAC naming of our compound:
For IUPAC naming we have to select the longest carbon chain which will be the parent group. So in ethylmethylamine the ethyl group has two carbons and the methyl group has one carbon hence ethyl becomes the parent carbon chain. Whenever amine is present with an alkyl group it is indicated by writing prefix N along with the alkyl group. Now we have methyl-substituted amine and hence the name of the compound becomes N-methylaminoethane where ethane being the parent chain.
From the above solution, it can be noted that two options match our solution.
And therefore the correct options are:
A.It is a dialkylamine
C.Its IUPAC name is N-methylaminoethane
Note:
for finding the correct solution if a structure is not known then by looking only at its name the solution of whether it is a dialkylamine or not can be found out. As it is clear that dialkylamine means amine having two alkyl groups attached to it. And for IUPAC naming just look into the parent carbon chain and to which group alkyl groups are attached.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




