
Which of the following statement is true regarding a carbohydrate having five carbons and an aldehyde group
(A) It can have $8$ stereoisomers
(B) It can have $4$ stereoisomers
(C) It can have $2$ stereoisomers
(D) All of the above
Answer
566.7k+ views
Hint: Carbohydrate is a biomolecule consisting of carbon, hydrogen, and oxygen atoms in the ratio of $2:1$ . Its empirical formula being ${C_m}{({H_2}O)_n}$. Also, the number of stereoisomers present in a molecule having n number of chiral atoms is given by the formula ${2^n}$ where n is the number of chiral atoms.
Complete step by step answer:
We have seen the carbohydrate with five carbon atoms and one aldehyde group. Chiral carbon is defined as the center along with four different valencies/groups that are present around it.
So we will draw the structure of the given configuration consisting of five carbon atoms and an aldehyde group.
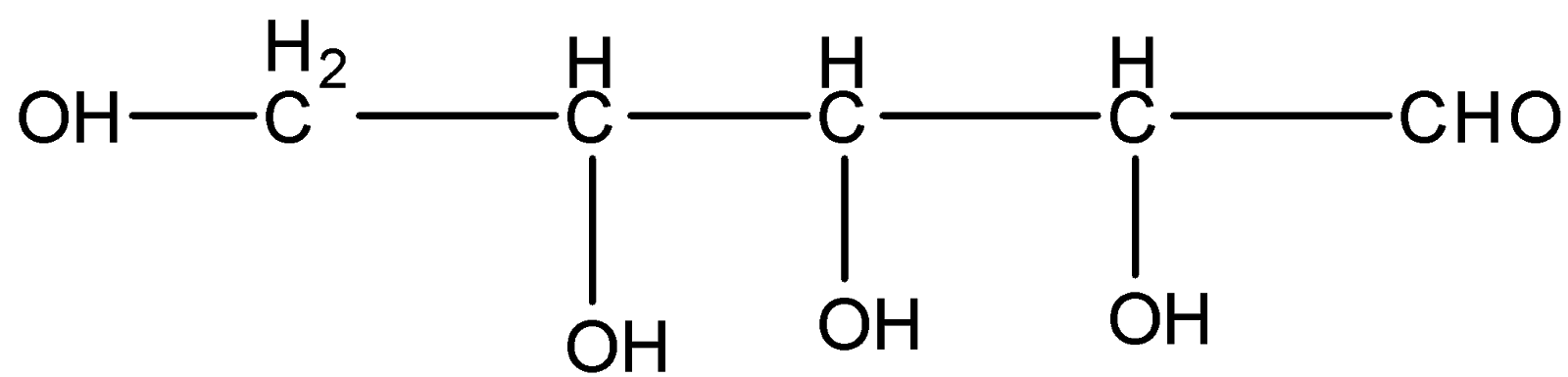
If we start numbering the carbon atoms from the aldehyde group then the three carbon atoms in succession with the aldehyde group are chiral because they have four different valencies attached to it.
Here we can see that there are three chiral carbons present in the molecule. Therefore according to the formula for a molecule consisting of n chiral carbon is given as ${2^{n}}$ where $n$ is the number of chiral carbon atoms
Therefore ${2^n} = {2^3} = 8$
So the total number of stereoisomers present in this molecule is eight.
So, the correct answer is Option A .
Note: In case of high symmetry this formula fails although for most of the cases it gives the right prediction of the number of stereoisomers. Also, stereoisomers are those compounds which are having the same composition as the starting compound but they differ in the orientation in space and they are classified into two parts such as enantiomers and diastereoisomers.
Complete step by step answer:
We have seen the carbohydrate with five carbon atoms and one aldehyde group. Chiral carbon is defined as the center along with four different valencies/groups that are present around it.
So we will draw the structure of the given configuration consisting of five carbon atoms and an aldehyde group.

If we start numbering the carbon atoms from the aldehyde group then the three carbon atoms in succession with the aldehyde group are chiral because they have four different valencies attached to it.
Here we can see that there are three chiral carbons present in the molecule. Therefore according to the formula for a molecule consisting of n chiral carbon is given as ${2^{n}}$ where $n$ is the number of chiral carbon atoms
Therefore ${2^n} = {2^3} = 8$
So the total number of stereoisomers present in this molecule is eight.
So, the correct answer is Option A .
Note: In case of high symmetry this formula fails although for most of the cases it gives the right prediction of the number of stereoisomers. Also, stereoisomers are those compounds which are having the same composition as the starting compound but they differ in the orientation in space and they are classified into two parts such as enantiomers and diastereoisomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




