
Which of the following shows the laboratory synthesis of Fluorobenzene $({{C}_{6}}{{H}_{5}}F)$?
A) By heating phenol with $HF$ and $KF$
B) From aniline by diazotization followed by heating the diazonium salt with $HB{{F}_{4}}$
C) By direct fluorination of benzene with ${{F}_{2}}$ gas
D) By reacting $PhBr$ with$NaF$solution
Answer
538.5k+ views
Hint: The answer is based on the concept of reaction mechanism of organic chemistry and also it reacts with the compound where the fluorine can attract the electrons as fluorine is the electronegative element.
Complete step-by-step answer:In the classes of organic chemistry, we have come across various named reactions and also some of the basic reactions like halogenations, addition reactions, substitution reactions and also the oxidation and reduction reactions.
Let us now see each of these options given one by one by reacting it with phenol and deduce the correct answer.
- In option A) When we heat phenol with fluorobenzene, we get the products which is as shown below,

- Here, since the fluoride ion is highly electronegative and it needs a group which is able to donate its electron and only then fluorine attaches to this compound.
- Here, even the acidity factor plays its role where acidity is measured based on the donation of electron and here since in$HF$, the more electronegative fluorine atom attracts the electron strongly from hydrogen, it does not donate the proton easily as$HF$is weak acid.
Thus, the reaction does not take place.
- In case of option B) The reaction is given a name Baltz-Schiemann reaction and it takes place as shown below:
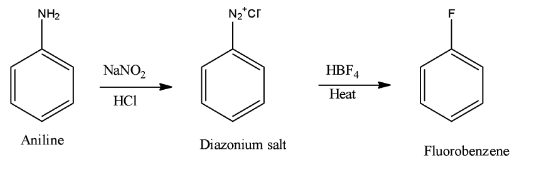
The reaction of diazonium salt with$HB{{F}_{4}}$yields fluorobenzene and this option is correct answer.
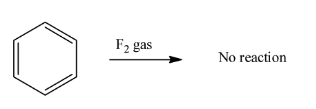
Now in option C) The fluorine atom is highly electronegative and for the homolytic division of fluorine gas that is${{F}_{2}}$needs higher energy and the stability of the reaction condition gets lower and therefore, the reaction is not possible. Therefore, this option is ruled out.
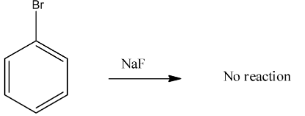
In case of option D) Treating of bromobenzene with$NaF$does not produce fluorobenzene as according to the Finkelstein reaction which is used for the halogen exchange reaction and since fluorine is highly electronegative than bromine, the reaction does not take place.
Therefore, the correct answer is option B).
Note:Note that Finkelstein reaction involves the exchange of the halogens that is alkyl or aryl bromide and also chloride with the sodium iodide solution and brominated compounds are more reactive than chlorinated compounds because the order of reactivity is iodide > bromides > chlorides > fluorides and therefore fluorides are not exchanged much here.
Complete step-by-step answer:In the classes of organic chemistry, we have come across various named reactions and also some of the basic reactions like halogenations, addition reactions, substitution reactions and also the oxidation and reduction reactions.
Let us now see each of these options given one by one by reacting it with phenol and deduce the correct answer.
- In option A) When we heat phenol with fluorobenzene, we get the products which is as shown below,

- Here, since the fluoride ion is highly electronegative and it needs a group which is able to donate its electron and only then fluorine attaches to this compound.
- Here, even the acidity factor plays its role where acidity is measured based on the donation of electron and here since in$HF$, the more electronegative fluorine atom attracts the electron strongly from hydrogen, it does not donate the proton easily as$HF$is weak acid.
Thus, the reaction does not take place.
- In case of option B) The reaction is given a name Baltz-Schiemann reaction and it takes place as shown below:

The reaction of diazonium salt with$HB{{F}_{4}}$yields fluorobenzene and this option is correct answer.
Now in option C) The fluorine atom is highly electronegative and for the homolytic division of fluorine gas that is${{F}_{2}}$needs higher energy and the stability of the reaction condition gets lower and therefore, the reaction is not possible. Therefore, this option is ruled out.

In case of option D) Treating of bromobenzene with$NaF$does not produce fluorobenzene as according to the Finkelstein reaction which is used for the halogen exchange reaction and since fluorine is highly electronegative than bromine, the reaction does not take place.

Therefore, the correct answer is option B).
Note:Note that Finkelstein reaction involves the exchange of the halogens that is alkyl or aryl bromide and also chloride with the sodium iodide solution and brominated compounds are more reactive than chlorinated compounds because the order of reactivity is iodide > bromides > chlorides > fluorides and therefore fluorides are not exchanged much here.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




