
Which of the following sequences would yield \[m - nitro\] chlorobenzene (Z) from benzene?
(A) \[Benzene\mathop \to \limits^{C{l_2}/FeC{l_3}} (X)\mathop \to \limits_{{H_2}S{O_4}}^{HN{O_3}} (Z)\] $$
(B) \[Benzene\mathop \to \limits^{{H_2}S{O_4}/HN{O_3}} (Z)\]
(C) \[Benzene\mathop \to \limits^{{H_2}S{O_4}/HN{O_3}} (X)\mathop \to \limits^{C{l_2}/FeC{l_3}} (Z)\]
(D) All of these
Answer
588.6k+ views
Hint:m-nitro chlorobenzene is an organic compound having formula ${C_6}{H_4}ClN{O_2}$. It is yellow in colour and acts as a precursor in many reactions. Benzene can be converted into m-nitro chlorobenzene by undergoing nitration and addition of chlorine.
Complete step by step answer:
As we know, benzene is ${C_6}{H_5}$, having structure as shown below
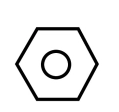
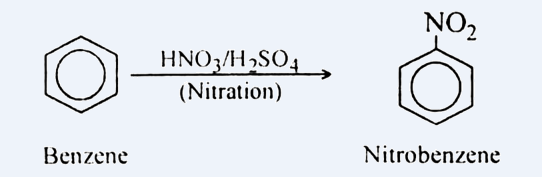
For converting it into m-nitro chlorobenzene, we need to add \[ - nitro\] and \[ - chloro\] groups in benzene. For the same, the reaction is carried out in two steps. In first step, we add nitrating mixture i.e. $HN{O_3}$ and ${H_2}S{O_4}$ to benzene, this step is known as nitration. It leads to the addition of $N{O_2}$ on Benzene. It can be shown as:
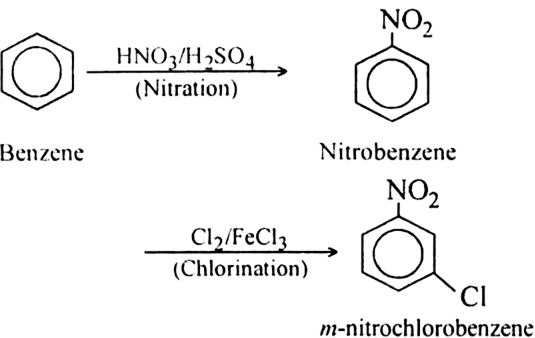
In the next step, $FeC{l_3}$ and $C{l_2}$ are added to nitrobenzene. $N{O_2}$ is an electron withdrawing, meta directing group. Therefore, it will add the chlorine at meta position, forming the desired product i.e. \[m - nitro\] chlorobenzene. The overall reaction is
The meta isomer of nitro chlorobenzene is not active towards nucleophilic substitution at chlorine center. m-nitro chlorobenzene can be reduced to \[3 - chloroaniline\;\] with \[Fe/HCl\] mixture, this reaction is called Bechamp reduction.
Hence, the correct option is C.
Additional information:
m-nitro chlorobenzene is also known as \[3 - nitro\] chlorobenzene. It’s IUPAC name is \[1 - chloro{\text{ }}3 - nitrobenzene\] . This compound generally does not have any direct application, but it acts as a precursor of other compounds such as \[3 - chloroaniline\] .
Note:
If we do the two above mentioned steps in reverse order i.e. firstly we add $FeC{l_3}$ and $C{l_2}$ into Benzene, it will form chlorobenzene. In the second step, if nitration is to be done we should first note that chlorine is the \[ortho/para\] directing group. Therefore, it will add the $N{O_2}$ at \[ortho/para\] position, forming \[o - nitro\] chlorobenzene or \[p - nitro\] p-nitro chlorobenzene but not m-nitro chlorobenzene.
Complete step by step answer:
As we know, benzene is ${C_6}{H_5}$, having structure as shown below

For converting it into m-nitro chlorobenzene, we need to add \[ - nitro\] and \[ - chloro\] groups in benzene. For the same, the reaction is carried out in two steps. In first step, we add nitrating mixture i.e. $HN{O_3}$ and ${H_2}S{O_4}$ to benzene, this step is known as nitration. It leads to the addition of $N{O_2}$ on Benzene. It can be shown as:

In the next step, $FeC{l_3}$ and $C{l_2}$ are added to nitrobenzene. $N{O_2}$ is an electron withdrawing, meta directing group. Therefore, it will add the chlorine at meta position, forming the desired product i.e. \[m - nitro\] chlorobenzene. The overall reaction is

The meta isomer of nitro chlorobenzene is not active towards nucleophilic substitution at chlorine center. m-nitro chlorobenzene can be reduced to \[3 - chloroaniline\;\] with \[Fe/HCl\] mixture, this reaction is called Bechamp reduction.
Hence, the correct option is C.
Additional information:
m-nitro chlorobenzene is also known as \[3 - nitro\] chlorobenzene. It’s IUPAC name is \[1 - chloro{\text{ }}3 - nitrobenzene\] . This compound generally does not have any direct application, but it acts as a precursor of other compounds such as \[3 - chloroaniline\] .
Note:
If we do the two above mentioned steps in reverse order i.e. firstly we add $FeC{l_3}$ and $C{l_2}$ into Benzene, it will form chlorobenzene. In the second step, if nitration is to be done we should first note that chlorine is the \[ortho/para\] directing group. Therefore, it will add the $N{O_2}$ at \[ortho/para\] position, forming \[o - nitro\] chlorobenzene or \[p - nitro\] p-nitro chlorobenzene but not m-nitro chlorobenzene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




