
Which of the following represents the Lewis structure of ${N_2}$ molecule?
A.
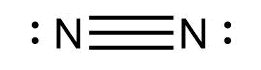
B.
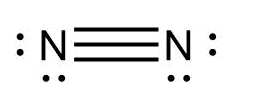
C.
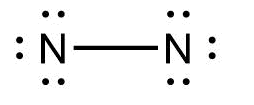
D.
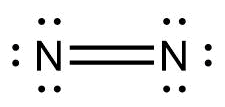
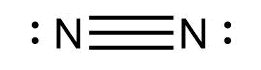

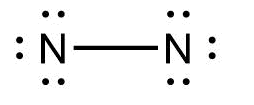
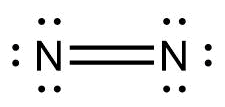
Answer
560.7k+ views
Hint: To answer this question, you must recall the Lewis dot structures for various elements and how to draw them. Lewis structures of chemical species (elements or compounds) are the graphic representations of the distribution of electrons around the constituent atoms. The electron pairs are represented using dots. The shared electron pairs depict the number of bonds formed between the atoms.
Complete step by step solution:
We know that the atomic number of nitrogen atoms is 7. It has five electrons in its valence shell. In order to complete the octets, each nitrogen needs three more electrons. Thus, a triple bond is formed between the two nitrogen atoms. The two remaining electrons are present as lone pairs on both the atoms. The Lewis dot structure for dinitrogen is
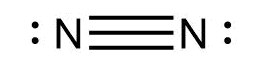
Thus, the correct answer is A.
Note: Lewis structures are commonly used for predicting the number and the types of bonds that are formed by an atom. One must follow some steps when drawing the Lewis structure for species which are:
A.First, we need to calculate the total number of valence electrons present in the molecules by adding the separate individual valencies of the constituent atoms.
B.In case of any anions present, extra electrons are added as dots in the structure and in case of cations, electrons are subtracted.
C.The least electronegative atom in the molecule must be placed as the central atom with all other atoms placed around it with a single bond.
D.Lone pairs must be assigned first to the most electronegative atoms.
E.Now draw multiple bonds if needed in order to complete the octet of each atom in the molecule.
Complete step by step solution:
We know that the atomic number of nitrogen atoms is 7. It has five electrons in its valence shell. In order to complete the octets, each nitrogen needs three more electrons. Thus, a triple bond is formed between the two nitrogen atoms. The two remaining electrons are present as lone pairs on both the atoms. The Lewis dot structure for dinitrogen is
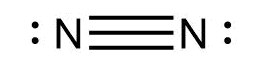
Thus, the correct answer is A.
Note: Lewis structures are commonly used for predicting the number and the types of bonds that are formed by an atom. One must follow some steps when drawing the Lewis structure for species which are:
A.First, we need to calculate the total number of valence electrons present in the molecules by adding the separate individual valencies of the constituent atoms.
B.In case of any anions present, extra electrons are added as dots in the structure and in case of cations, electrons are subtracted.
C.The least electronegative atom in the molecule must be placed as the central atom with all other atoms placed around it with a single bond.
D.Lone pairs must be assigned first to the most electronegative atoms.
E.Now draw multiple bonds if needed in order to complete the octet of each atom in the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




