
Which of the following represents the anomer of the compound shown?
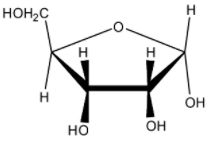
(A)
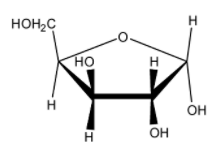
(B)
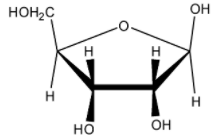
(C)
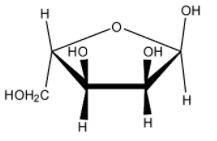
(D) None of these




Answer
587.7k+ views
Hint: Anomers are stereoisomers (diastereoisomers) which differ in their respective configurations only around the ${ C }_{ 1 }$ carbon while the configuration of the rest of the carbons remains the same.
Complete answer:
The structure of a carbohydrate can be drawn in an open chain form. Given below is the open chain form of D-Glucose:
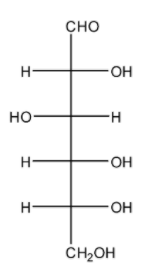
But there are certain limitations to the open chain structure such as:
-Even though the open chain structure has a –CHO group, even then the D-Glucose does not undergo reactions such as 2,4-DNP test, it does not form the aldehyde-ammonia adduct as well as the ${ NaHSO }_{ 3 }$ addition product.
-D-Glucose exists in two stereoisomeric forms: $\alpha $ -glucose and $\beta $-glucose and they undergo mutarotation in aqueous medium.
Due to these limitations, it was suggested that the monosaccharides do not have a free aldehyde or ketone group but rather a cyclic hemiacetal or hemiketal structure. The –OH groups (attached to ${ C }_{ 4 }$ or ${ C }_{ 5 }$ in aldohexoses and ${ C }_{ 5 }$ or ${ C }_{ 6}$ in ketohexoses) combine with the aldehyde or the keto group in order to form the intramolecular hemiacetal or hemiketal giving rise to five or six-membered cyclic structures containing an oxygen atom in the ring. If it is a five membered ring then it is called furanose form and if it is a six membered ring then it is called the pyranose form.
Therefore in the hemiacetal form of D-Glucose, there could be two structures in which the stereochemistry at the ${ C }_{ 1 }$ is different since it has become chiral in the cyclic form. This is illustrated in the following diagram:
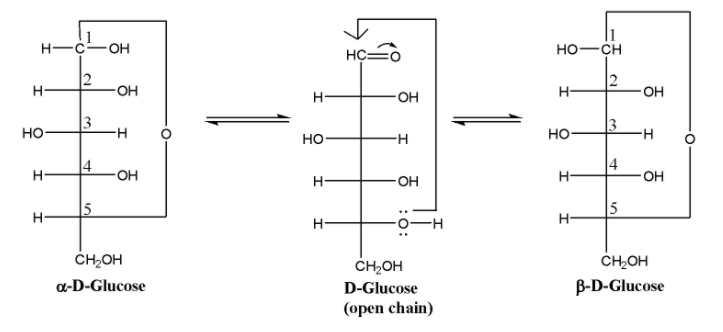
In $\alpha $-D-Glucose, the –OH group at the ${ C }_{ 1 }$ carbon is towards the right while in $\beta $-D-Glucose, it is towards the left. Hence these two are stereoisomers which differ in the configuration only around the ${ C }_{ 1 }$ and are called anomers while the ${ C }_{ 1 }$ carbon is called the anomeric carbon.
So, in the given compound:
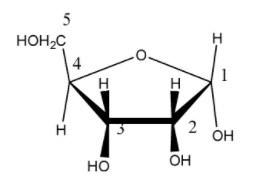
The answer will be that compound which will differ in its configuration only at the ${ C }_{ 1 }$ carbon while the configuration of the rest of the carbon atoms will be the same.
Hence the correct answer is (B) .

Note:
Pentoses and hexoses always exist in the pyranose form in the free state. But in the combined state, some monosaccharides such as ribose, 2-deoxyribose, fructose etc. generally exist in the furanose form. Trioses and tetroses exist in the open chain form.
Complete answer:
The structure of a carbohydrate can be drawn in an open chain form. Given below is the open chain form of D-Glucose:

But there are certain limitations to the open chain structure such as:
-Even though the open chain structure has a –CHO group, even then the D-Glucose does not undergo reactions such as 2,4-DNP test, it does not form the aldehyde-ammonia adduct as well as the ${ NaHSO }_{ 3 }$ addition product.
-D-Glucose exists in two stereoisomeric forms: $\alpha $ -glucose and $\beta $-glucose and they undergo mutarotation in aqueous medium.
Due to these limitations, it was suggested that the monosaccharides do not have a free aldehyde or ketone group but rather a cyclic hemiacetal or hemiketal structure. The –OH groups (attached to ${ C }_{ 4 }$ or ${ C }_{ 5 }$ in aldohexoses and ${ C }_{ 5 }$ or ${ C }_{ 6}$ in ketohexoses) combine with the aldehyde or the keto group in order to form the intramolecular hemiacetal or hemiketal giving rise to five or six-membered cyclic structures containing an oxygen atom in the ring. If it is a five membered ring then it is called furanose form and if it is a six membered ring then it is called the pyranose form.
Therefore in the hemiacetal form of D-Glucose, there could be two structures in which the stereochemistry at the ${ C }_{ 1 }$ is different since it has become chiral in the cyclic form. This is illustrated in the following diagram:

In $\alpha $-D-Glucose, the –OH group at the ${ C }_{ 1 }$ carbon is towards the right while in $\beta $-D-Glucose, it is towards the left. Hence these two are stereoisomers which differ in the configuration only around the ${ C }_{ 1 }$ and are called anomers while the ${ C }_{ 1 }$ carbon is called the anomeric carbon.
So, in the given compound:

The answer will be that compound which will differ in its configuration only at the ${ C }_{ 1 }$ carbon while the configuration of the rest of the carbon atoms will be the same.
Hence the correct answer is (B) .

Note:
Pentoses and hexoses always exist in the pyranose form in the free state. But in the combined state, some monosaccharides such as ribose, 2-deoxyribose, fructose etc. generally exist in the furanose form. Trioses and tetroses exist in the open chain form.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




