
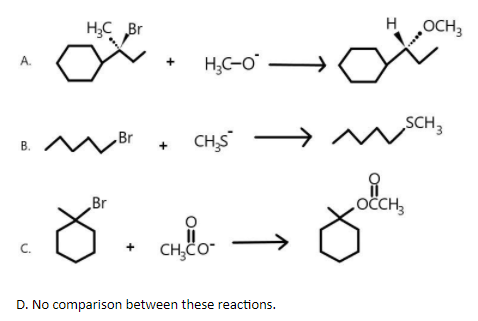
Which of the following reactions will go faster if the concentration of nucleophiles is increased?

Answer
558.6k+ views
Hint: As we know that the given reactions are undergoing substitution nucleophilic unimolecular and bimolecular reactions. And we also know that the rate of a reaction is directly proportional to the concentration of a given nucleophile.
Complete step-by-step answer:
As we know that the first and second reactions are undergoing substitution nucleophile bimolecular reaction i.e. ${S_N}2$ reaction where a single step bimolecular reaction is taking place in which the incoming nucleophile is attacking the carbon atom of the substrate in opposite direction to the outgoing nucleophile.
But in the third reaction, substitution nucleophile unimolecular reaction i.e. ${S_N}1$ reaction is taking place where two step reaction happens. The first step involves the slow ionisation of substrate and the second step involves the reaction between the carbocation so formed and the nucleophile. As show below, bromine will first get removed and then methoxy group will be added as nucleophile.
We are also aware with the fact that rate of reaction in ${S_N}2$ reaction is directly proportional to the concentration of a given nucleophile.Therefore, we can say that the first and second reactions will go faster if nucleophile concentration is increased.
Hence, the correct answers are (A) and (B).
Note: Always remember that the concentration is the sole factor on which the rate of a reaction is dependent in case of ${S_N}2$ reactions only whereas in case of ${S_N}1$ reaction, the rate of reaction depends upon the concentration of alkyl halide only and it is independent of the concentration of nucleophile. Further, the higher the stability of the carbocation, higher will be the ease of carbocation formation from alkyl halide and faster will be the rate of reaction.
Complete step-by-step answer:
As we know that the first and second reactions are undergoing substitution nucleophile bimolecular reaction i.e. ${S_N}2$ reaction where a single step bimolecular reaction is taking place in which the incoming nucleophile is attacking the carbon atom of the substrate in opposite direction to the outgoing nucleophile.
But in the third reaction, substitution nucleophile unimolecular reaction i.e. ${S_N}1$ reaction is taking place where two step reaction happens. The first step involves the slow ionisation of substrate and the second step involves the reaction between the carbocation so formed and the nucleophile. As show below, bromine will first get removed and then methoxy group will be added as nucleophile.
We are also aware with the fact that rate of reaction in ${S_N}2$ reaction is directly proportional to the concentration of a given nucleophile.Therefore, we can say that the first and second reactions will go faster if nucleophile concentration is increased.
Hence, the correct answers are (A) and (B).
Note: Always remember that the concentration is the sole factor on which the rate of a reaction is dependent in case of ${S_N}2$ reactions only whereas in case of ${S_N}1$ reaction, the rate of reaction depends upon the concentration of alkyl halide only and it is independent of the concentration of nucleophile. Further, the higher the stability of the carbocation, higher will be the ease of carbocation formation from alkyl halide and faster will be the rate of reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




