
Which of the following reactions is possible?
A) \[\text{M}{{\text{e}}_{\text{3}}}\text{C -- ONa+ M}{{\text{e}}_{\text{3}}}\text{C -- Br }\to \text{ M}{{\text{e}}_{\text{3}}}\text{C -- O -- CM}{{\text{e}}_{\text{3}}}\]
B \[\text{M}{{\text{e}}_{\text{2}}}\text{CH -- ONa + M}{{\text{e}}_{\text{2}}}\text{CHBr }\to \text{ M}{{\text{e}}_{\text{2}}}\text{CH -- O -- CHM}{{\text{e}}_{\text{2}}}\]
C \[\text{PhONa + PhBr }\to \text{ PhOPh}\]
D \[\text{PhONa+C}{{\text{H}}_{\text{3}}}\text{Br}\to \text{PhOC}{{\text{H}}_{\text{3}}}\]
Answer
589.5k+ views
Hint: Symmetrical or unsymmetrical Ethers can be prepared by Williamson's synthesis. The alkyl halides $\text{R-X}$ undergo the reaction with sodium alkoxide $\text{R }\!\!'\!\!\text{ -ONa}$ to prepare ethers $\text{R }\!\!'\!\!\text{ -O-R}$. The general formula is as:
$\begin{align}
& \text{R }\!\!'\!\!\text{ -}{{\text{O}}^{\text{-}}}\text{ -R }\xrightarrow{\Delta }\text{R }\!\!'\!\!\text{ -O-R+NaX} \\
& \text{ (Ether)} \\
\end{align}$
Complete step by step answer:
Ethers are having a functional group$\text{-O-}$. The general formula for the ethers is $\text{R-O-R}$ where R may be alkyl or aryl groups.
Ethers can be prepared from the alkyl halides. This is one of the best methods for preparing symmetrical and unsymmetrical ethers in the laboratory. In this method, an alkyl halide is treated with suitable sodium alkoxide prepared by the action of sodium on suitable alcohol. The reaction involves the nucleophilic substitution $\text{(}{{\text{S}}_{\text{N}}}\text{2)}$ attack of an alkoxide ion on primary alkyl halide.
$\begin{align}
& \text{2R }\!\!'\!\!\text{ OH+2Na}\to \text{ 2R }\!\!'\!\!\text{ }{{\text{O}}^{\text{-}}}\text{-N}{{\text{a}}^{\text{+}}}\text{ }+\text{ }{{\text{H}}_{\text{2}}} \\
& \text{ (Sodium Alkoxide)} \\
\end{align}$
Let's have a look on option,
The tert-butyl bromide is an ${{3}^{0}}$ alkyl halide. It reacts with the sodium tert-butoxide. It prefers to undergo the elimination reaction rather than the substitution reaction. Therefore, isobutylene is obtained as the product rather than the di-tert butyl ether. Thus di-tert butyl ether cannot undergo the Williamson synthesis.
\[\begin{matrix}
{} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} \\
{} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & | & {} & {} & {} \\
\text{C}{{\text{H}}_{\text{3}}}- & \text{C} & - & \text{C}{{\text{H}}_{\text{3}}} & \text{+} & \text{N}{{\text{a}}^{\text{+}}}\text{O} & - & \text{C} & - & \text{C}{{\text{H}}_{\text{3}}} & \to & \text{C}{{\text{H}}_{\text{3}}} & - & \text{C} & = & \text{C}{{\text{H}}_{\text{2}}} & + & \text{C}{{\text{H}}_{\text{3}}} & - & \text{C} & - & \text{OH} & {} \\
{} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & {} & {} & {} & {} & {} & {} & | & {} & {} & {} \\
{} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & {} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} \\
\end{matrix}\]
Due to steric hindrance, the secondary alkyl halide prefers to undergo the elimination reaction rather than the substitution given a mixture of both elimination (alkene) and substitution (ether) product. Thus option (B) is not true. The reaction is $\text{(}{{\text{S}}_{\text{N}}}\text{2)}$ a reaction therefore the highly substituted or secondary alkyl halide does not undergo reaction readily.
The aryl halides and aryl halides like sodium phenoxide and bromobenzene cannot undergo the reaction to for diaryl ethers this is because aryl halides or bromobenzene do not undergo nucleophilic substitution reaction easily.
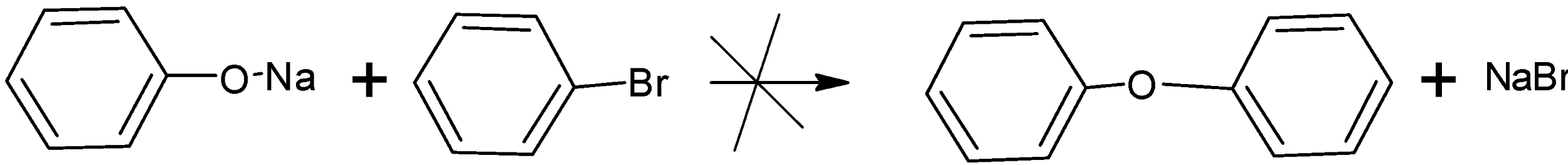
Let's have a look at option D).the alkyl aryl ethers can be easily prepared by treating the phenoxide with the aryl halide. Here the sodium phenoxide reacts with the bromomethane to give anisole or phenyl methyl ether. This is because of the Williamson synthesis reaction.
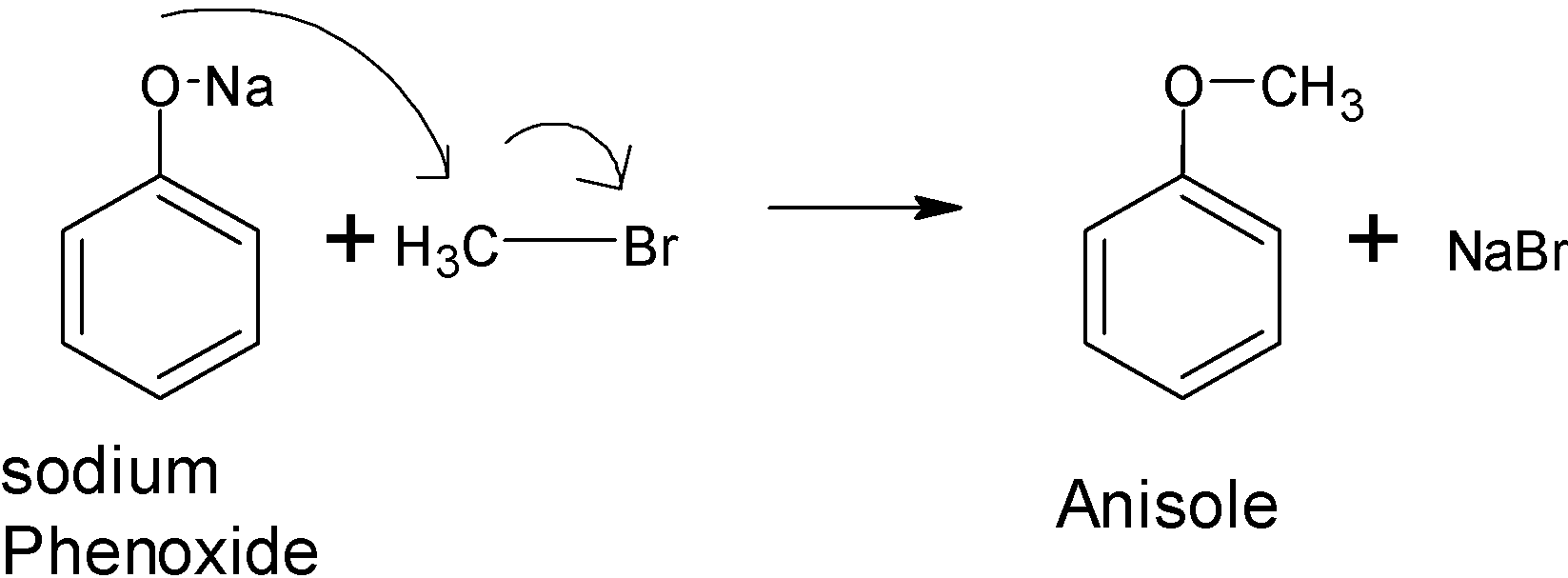
Option (A), (B) and (C) are not true. So, the correct answer is “Option D”.
Note: It may be noted that the phenols are more acidic than alcohols and therefore, these can be converted to sodium phenoxide by treating with $\text{NaOH}$. In alcohols, metallic sodium is used to convert alcohols to alkoxide ions. Primary halides are preferred to use as they are more reactive.
$\begin{align}
& \text{R }\!\!'\!\!\text{ -}{{\text{O}}^{\text{-}}}\text{ -R }\xrightarrow{\Delta }\text{R }\!\!'\!\!\text{ -O-R+NaX} \\
& \text{ (Ether)} \\
\end{align}$
Complete step by step answer:
Ethers are having a functional group$\text{-O-}$. The general formula for the ethers is $\text{R-O-R}$ where R may be alkyl or aryl groups.
Ethers can be prepared from the alkyl halides. This is one of the best methods for preparing symmetrical and unsymmetrical ethers in the laboratory. In this method, an alkyl halide is treated with suitable sodium alkoxide prepared by the action of sodium on suitable alcohol. The reaction involves the nucleophilic substitution $\text{(}{{\text{S}}_{\text{N}}}\text{2)}$ attack of an alkoxide ion on primary alkyl halide.
$\begin{align}
& \text{2R }\!\!'\!\!\text{ OH+2Na}\to \text{ 2R }\!\!'\!\!\text{ }{{\text{O}}^{\text{-}}}\text{-N}{{\text{a}}^{\text{+}}}\text{ }+\text{ }{{\text{H}}_{\text{2}}} \\
& \text{ (Sodium Alkoxide)} \\
\end{align}$
Let's have a look on option,
The tert-butyl bromide is an ${{3}^{0}}$ alkyl halide. It reacts with the sodium tert-butoxide. It prefers to undergo the elimination reaction rather than the substitution reaction. Therefore, isobutylene is obtained as the product rather than the di-tert butyl ether. Thus di-tert butyl ether cannot undergo the Williamson synthesis.
\[\begin{matrix}
{} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} \\
{} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & | & {} & {} & {} \\
\text{C}{{\text{H}}_{\text{3}}}- & \text{C} & - & \text{C}{{\text{H}}_{\text{3}}} & \text{+} & \text{N}{{\text{a}}^{\text{+}}}\text{O} & - & \text{C} & - & \text{C}{{\text{H}}_{\text{3}}} & \to & \text{C}{{\text{H}}_{\text{3}}} & - & \text{C} & = & \text{C}{{\text{H}}_{\text{2}}} & + & \text{C}{{\text{H}}_{\text{3}}} & - & \text{C} & - & \text{OH} & {} \\
{} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & \text{ }\!\!|\!\!\text{ } & {} & {} & {} & {} & {} & {} & {} & {} & {} & {} & {} & | & {} & {} & {} \\
{} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} & {} & {} & {} & {} & {} & {} & {} & {} & \text{C}{{\text{H}}_{\text{3}}} & {} & {} & {} \\
\end{matrix}\]
Due to steric hindrance, the secondary alkyl halide prefers to undergo the elimination reaction rather than the substitution given a mixture of both elimination (alkene) and substitution (ether) product. Thus option (B) is not true. The reaction is $\text{(}{{\text{S}}_{\text{N}}}\text{2)}$ a reaction therefore the highly substituted or secondary alkyl halide does not undergo reaction readily.
The aryl halides and aryl halides like sodium phenoxide and bromobenzene cannot undergo the reaction to for diaryl ethers this is because aryl halides or bromobenzene do not undergo nucleophilic substitution reaction easily.

Let's have a look at option D).the alkyl aryl ethers can be easily prepared by treating the phenoxide with the aryl halide. Here the sodium phenoxide reacts with the bromomethane to give anisole or phenyl methyl ether. This is because of the Williamson synthesis reaction.

Option (A), (B) and (C) are not true. So, the correct answer is “Option D”.
Note: It may be noted that the phenols are more acidic than alcohols and therefore, these can be converted to sodium phenoxide by treating with $\text{NaOH}$. In alcohols, metallic sodium is used to convert alcohols to alkoxide ions. Primary halides are preferred to use as they are more reactive.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




