
Which of the following reactions is feasible for the preparation of sec-butyl propyl ether?
(A) Williamson’s synthesis with
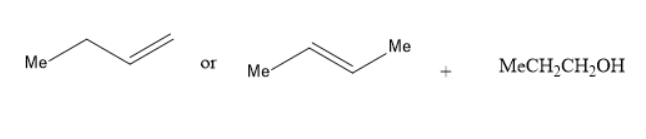
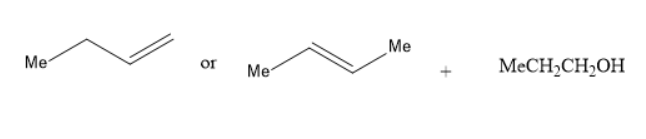
(B) Alkoxymercuration-demercuration of
(C) Alkoxymercuration-demercuration of propene $ + MeC{H_2}CH\left( {Me} \right) - OH $
(D) Intermolecular dehydration of sec-butyl alcohol and isopropyl alcohol.


Answer
480.6k+ views
Hint: Alkoxymercuration-demercuration reaction is a reaction in which the alkanes react with alcohol in presence of mercury acetate to yield alkoxy mercury intermediate, upon reduction with sodium borohydride gives the organic compound ether.
Complete answer:
Williamson’s ether synthesis is a reaction in which alcohol reacts with organic halide means halogen compounds to yield an ether. In this reaction, the hydrochloric acid molecules can be eliminated. In the given reagents, an alkene is given but not halogen compounds. Thus, it does not give either.
In the reaction of alkoxy mercuration-demercuration, $ 1 - $ butene or $ 2 - $ butene reacts with propanol to yield an ether. The chemical reaction is as follows:
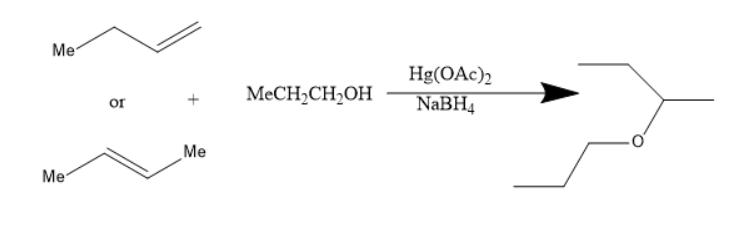
The product in the above reaction is sec-butyl propyl ether. Thus, this reaction is feasible for the preparation of sec-butyl propyl ether.
The reaction of alkoxy mercuration-demercuration of propene $ + MeC{H_2}CH\left( {Me} \right) - OH $ gives the product which is sec-butyl isopropyl ether but not sec-butyl propyl ether. Thus, it is not a correct answer.
The intermolecular dehydration of sec-butyl alcohol and isopropyl alcohol gives sec-butyl isopropyl ether but not sec-butyl propyl ether.
Thus, option B is the correct answer.
Note:
Ethers are the organic compounds consisting of oxygen atoms between the two alkyl groups or carbon atoms that are a part of an alkyl group. These compounds can be prepared from both Williamson’s ether synthesis and alkoxy mercuration-demercuration. But the products were different.
Complete answer:
Williamson’s ether synthesis is a reaction in which alcohol reacts with organic halide means halogen compounds to yield an ether. In this reaction, the hydrochloric acid molecules can be eliminated. In the given reagents, an alkene is given but not halogen compounds. Thus, it does not give either.
In the reaction of alkoxy mercuration-demercuration, $ 1 - $ butene or $ 2 - $ butene reacts with propanol to yield an ether. The chemical reaction is as follows:

The product in the above reaction is sec-butyl propyl ether. Thus, this reaction is feasible for the preparation of sec-butyl propyl ether.
The reaction of alkoxy mercuration-demercuration of propene $ + MeC{H_2}CH\left( {Me} \right) - OH $ gives the product which is sec-butyl isopropyl ether but not sec-butyl propyl ether. Thus, it is not a correct answer.
The intermolecular dehydration of sec-butyl alcohol and isopropyl alcohol gives sec-butyl isopropyl ether but not sec-butyl propyl ether.
Thus, option B is the correct answer.
Note:
Ethers are the organic compounds consisting of oxygen atoms between the two alkyl groups or carbon atoms that are a part of an alkyl group. These compounds can be prepared from both Williamson’s ether synthesis and alkoxy mercuration-demercuration. But the products were different.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




