
Which of the following reactions is called saponification?
(a) $C{{H}_{3}}COOC{{H}_{3}}+O{{H}^{-}}\to C{{H}_{3}}CO{{O}^{-}}+C{{H}_{3}}OH$
(b) $C{{H}_{3}}CO{{O}^{-}}N{{a}^{+}}\xrightarrow{HCl}C{{H}_{3}}COOH+NaCl$
(c)
(d) $C{{H}_{3}}COOH+C{{H}_{3}}C{{H}_{2}}OH\to C{{H}_{3}}COO{{H}_{2}}C{{H}_{3}}$

Answer
582.9k+ views
Hint: Soaps are the sodium or potassium salts of long chain of higher fatty acids and are formed when fats and oils (esters of higher fatty acids and glycerol) is treated with an alkali and this process is known as the saponification. Now identify the reaction.
Complete step by step answer:
By the term saponification we mean the process of making soap by the hydrolysis of fats and oils with alkalis. When oil and fats (glycerides) are heated with a solution of sodium hydroxide , they break down to sodium salt of the respective fatty acids(called soap) and glycerol.
The reaction occurs as:
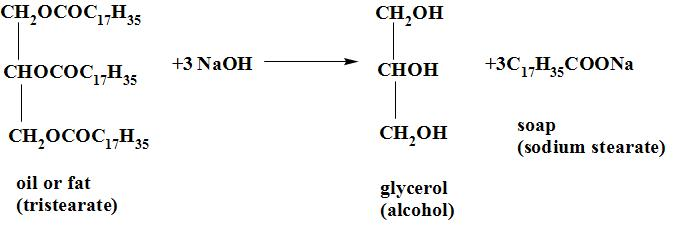
The soap is separated from the solution by the addition of common salt (NaCl). Salt is added to it to decrease the solubility of soap and it helps to precipitate out from the aqueous solution. soap is lighter than water and it floats on the surface from where it is removed. The solution remaining behind floats on the surface from where it is removed. The solution remaining behind contains glycerol and sodium chloride. Glycerol is recovered from the solution as it is a very useful chemical and is used in the drugs , cosmetics, explosives and paints.
So, from the above reaction $C{{H}_{3}}COOC{{H}_{3}}+O{{H}^{-}}\to C{{H}_{3}}CO{{O}^{-}}+C{{H}_{3}}OH$ is called as the saponification reaction.
So, the correct answer is “Option A”.
Note: Soap is a good cleansing agent and is 100% biodegradable i.e. microorganisms present in sewage water can completely oxidize soap and thus, the soaps do not cause any pollution problems.
Complete step by step answer:
By the term saponification we mean the process of making soap by the hydrolysis of fats and oils with alkalis. When oil and fats (glycerides) are heated with a solution of sodium hydroxide , they break down to sodium salt of the respective fatty acids(called soap) and glycerol.
The reaction occurs as:

The soap is separated from the solution by the addition of common salt (NaCl). Salt is added to it to decrease the solubility of soap and it helps to precipitate out from the aqueous solution. soap is lighter than water and it floats on the surface from where it is removed. The solution remaining behind floats on the surface from where it is removed. The solution remaining behind contains glycerol and sodium chloride. Glycerol is recovered from the solution as it is a very useful chemical and is used in the drugs , cosmetics, explosives and paints.
So, from the above reaction $C{{H}_{3}}COOC{{H}_{3}}+O{{H}^{-}}\to C{{H}_{3}}CO{{O}^{-}}+C{{H}_{3}}OH$ is called as the saponification reaction.
So, the correct answer is “Option A”.
Note: Soap is a good cleansing agent and is 100% biodegradable i.e. microorganisms present in sewage water can completely oxidize soap and thus, the soaps do not cause any pollution problems.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




